Calcium oxide-based bimetallic composite material as well as preparation method and application thereof
A composite material, calcium oxide technology, applied in the field of preparation, calcium oxide-based bimetallic composite materials, can solve the problem of severe methanation by-products, achieve the effect of solving sintering failure, preventing sintering, and enhancing the dual functions of adsorption and catalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
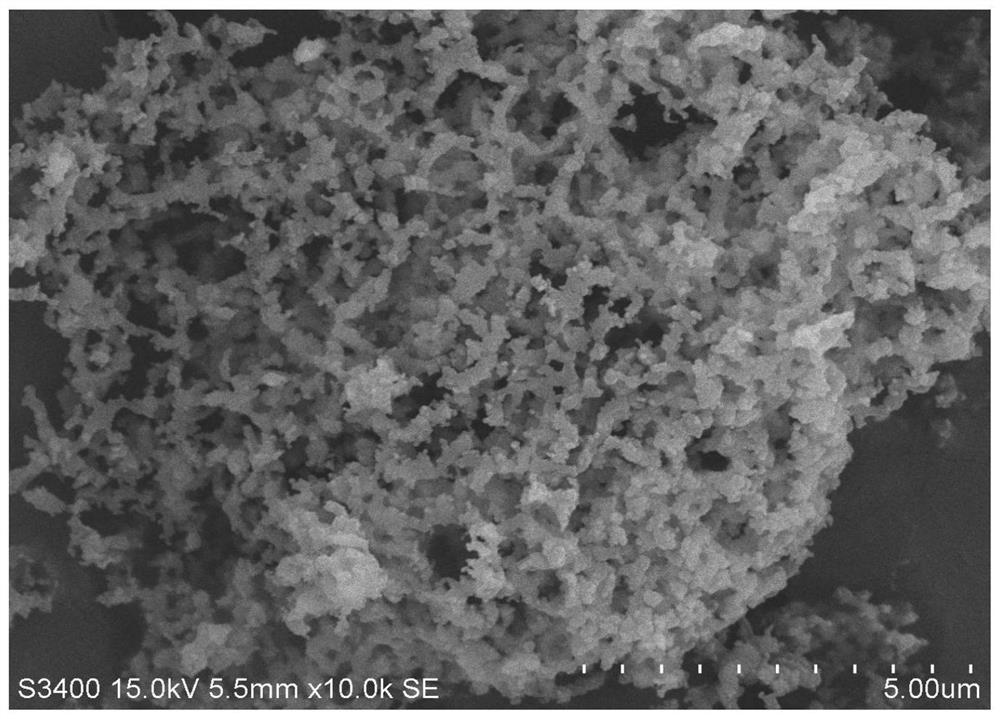
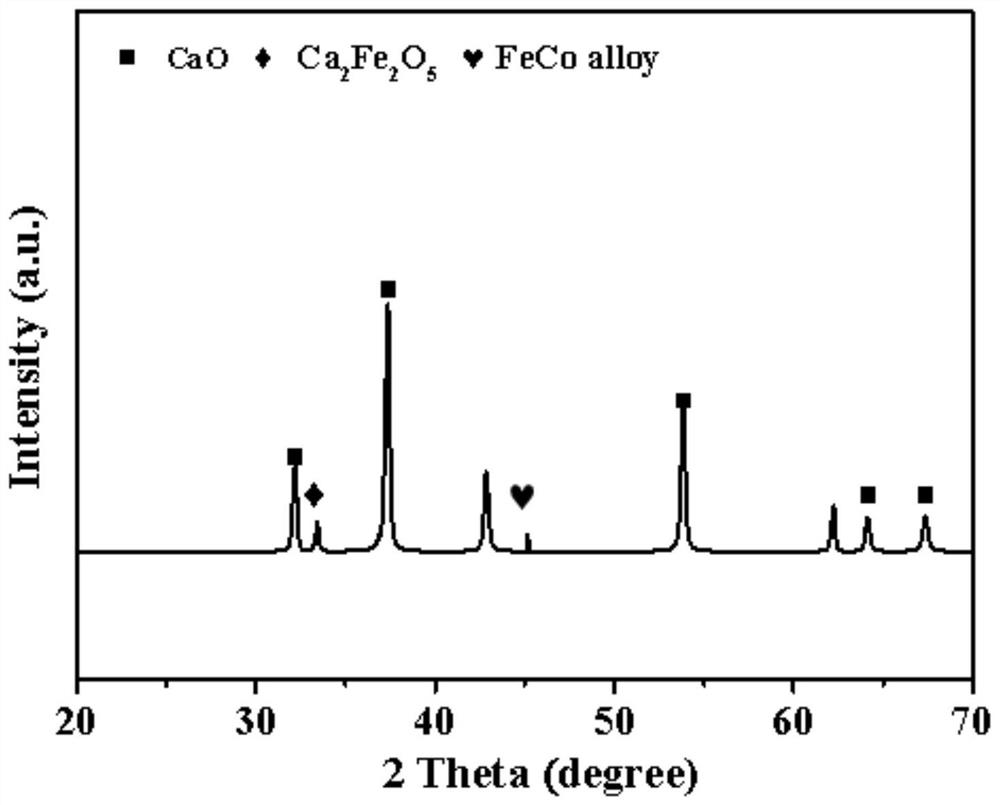
[0045] Weigh 8.433gCa(NO 3 ) 2 4H 2 O is dissolved in 40mL of deionized water to obtain a solution, after stirring evenly, add organic template agent 6.5gC 6 h 8 o 7 ·H 2 O and 1.0g cetyltrimethylammonium bromide, continue to stir until fully dissolved, continue to add 0.72gFe(NO 3 ) 2 9H 2 O and 0.49gCo(NO 3 ) 2 ·6H 2 O, stir evenly to obtain a mixed solution. Stir the mixed solution in a constant temperature water bath at 90°C for 5 hours to obtain a translucent gel, cool to room temperature, place the gel in an oven at 120°C, heat and dry for 12 hours to obtain a xerogel. Finally, the dry gel was calcined at 800°C for 4 hours, then ground, pressed into tablets, and crushed to obtain a composite material Fe with a particle size of 0.2-0.3 mm. 5 co 5 CaO, the mass ratio of CaO:FeCo metal oxide (calculated according to the metal salt precursor) in the composite material is 1:0.1, wherein Fe%:Co%=1:1.
[0046] The prepared adsorption-catalysis bifunctional composi...
Embodiment 2
[0051] Weigh 5.24gCaCl 2 2H 2 O was dissolved in 40mL of deionized water to obtain a solution. After stirring evenly, 10g of organic template agent P123 was added, and the stirring was continued until it was fully dissolved, and 0.97g of Fe(NO 3 ) 2 9H 2 O and 0.32gCo(NO 3 ) 2 ·6H 2 O, stir evenly to obtain a mixed solution. The mixed solution was stirred in a constant temperature water bath at 80°C for 5 hours to obtain a translucent gel, cooled to room temperature, placed in an oven at 120°C, heated and dried for 14 hours to obtain a xerogel. Finally, the dry gel was calcined at 800°C for 5 hours, then ground, pressed into tablets, and crushed to obtain a composite material Fe with a particle size of 0.2-0.3 mm. 10 co 5 CaO, the mass ratio of CaO:FeCo metal oxide in the composite material is 1:0.15, wherein Fe%:Co%=2:1.
[0052] Using the same analytical method as in Example 1, the results show that the prepared adsorption-catalysis dual-functional porous composite ...
Embodiment 3
[0054] Weigh 8.43gCa(NO 3 ) 2 4H 2 O was dissolved in 40mL of deionized water to obtain a solution. After stirring evenly, 5g of organic templating agent hexadecyltrimethylammonium bromide and 10g of ammonium oxalate were added, and stirring was continued until fully dissolved, and 0.47g of Fe(NO 3 ) 2 9H 2 O and 0.22gCo(NO 3 ) 2 ·6H 2 O, stir evenly to obtain a mixed solution. Stir the mixed solution in a constant temperature water bath at 100°C for 6 hours to obtain a translucent gel, cool to room temperature, put the gel in an oven at 130°C, heat and dry for 16 hours to obtain a xerogel. Finally, the dry gel was calcined at 900°C for 4 hours, then ground, pressed into tablets, and crushed to obtain a composite material Fe with a particle size of 0.2-0.3 mm. 3 co 2 CaO, the mass ratio of CaO:FeCo metal oxide in the composite material is 1:0.05, wherein Fe%:Co%=3:2.
[0055] Using the same analytical method as in Example 1, the results show that the prepared adsorpt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com