Collagen for preparing hydrogel and preparation method of collagen
A collagen and hydrogel technology, applied in the field of bioengineering, can solve the problems of poor adhesion and unsuitable for in vivo application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of embodiment 1 vector plasmid
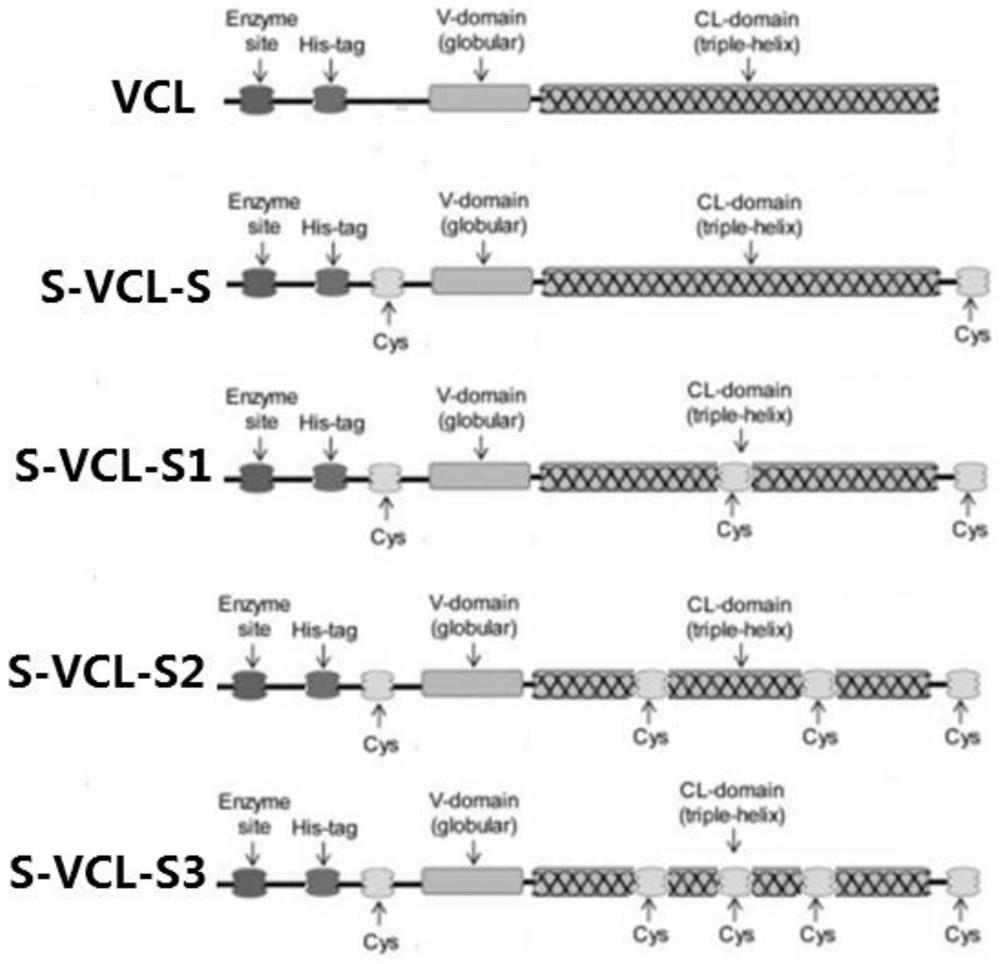
[0034] The control protein sequence VCL was designed according to the codon preference of the expression host Escherichia coli BL21 (DE3), and its specific sequence is shown in SEQ ID No.5. Then according to figure 1 The design mechanism shown in the sequence design sequence S-VCL-S, S-VCL-S1, S-VCL-S2, S-VCL-S3, synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd., and connected to the pET-28a vector plasmid.
[0035] A collagen S-VCL-S for preparing a hydrogel, the sequence of which is shown in SEQ ID No.1, and cysteines are respectively connected at both ends;
[0036] A collagen S-VCL-S1 used for preparing hydrogel, its sequence is shown in SEQ ID No.2, except that cysteine is connected at both ends, and cysteine is inserted into the sequence at the same time.
[0037] A collagen S-VCL-S2 used for preparing hydrogel, its sequence is shown in SEQ ID No.3, except that cysteines are connected at both ends, and two cys...
Embodiment 2
[0039] The preparation of embodiment 2 collagen
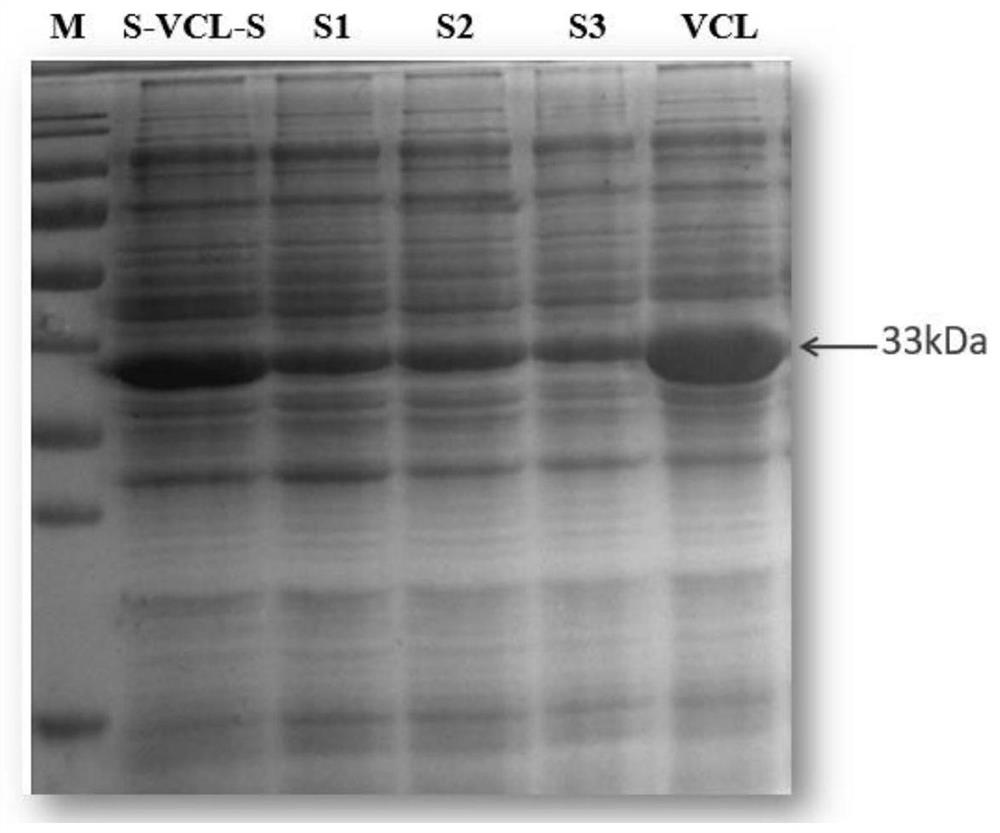
[0040] The designed sequence contains the Nco I restriction site and the Bam HI restriction site, and the recombinant plasmid pET-28a containing the polymer gene of the target length was obtained through double restriction screening verification. The verification results are as follows: figure 2 As shown, it proves that it successfully connects the target gene sequence.
[0041] (1) Take Escherichia coli BL21(DE3) competent cells and thaw on ice, add all 20 μL of recombinant plasmids to 100 μL competent cells, gently pipette evenly, and place on ice for 30 minutes. After the ice bath, the competent cells were heat-shocked in a water bath at 42°C for 90 seconds, and quickly placed on ice to cool for 2 minutes after removal. Then, add 500 μL of fresh LB medium without antibiotics to the test tube, and incubate in a constant temperature shaker at 37°C for 1 hour, then spread the bacterial solution on the LB plate containing Kann...
Embodiment 3
[0046] The preparation of embodiment 3 collagen hydrogels
[0047] Take 90 μL of 4.5% w / v one of the four collagen solutions at the bottom of the glass tube, add 10 μL of 0.1% H 2 o 2 Mix gently; place in a 37°C water bath and incubate for 30min. The VCL protein without cysteine was used as the control group, and 0.1% H 2 o 2 , and incubated at 37°C for 30min. After the incubation is complete, remove the glass tube and place it upside down. If the polymer molecules are cross-linked to form a hydrogel, its fluidity is limited and still exists at the bottom of the glass tube; otherwise, it will flow down the tube wall, which can be used to detect whether the gel is formed or not.
[0048] The results showed that VCL could not successfully form hydrogels, while S-VCL-S, S-VCL-S1, S-VCL-S2, and S-VCL-S3 could successfully prepare hydrogels.
[0049] Figure 5 It is the SEM picture of S-VCL-S collagen hydrogel. It can be seen from the picture that the hydrogel exhibits a u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com