Bacterial vaginosis diagnostic
An activity and indicator technology, applied in disease diagnosis, instruments, peptides, etc., can solve problems such as lack of sensitivity and lack of accuracy of tests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0330] Example 1: Assay Chemistry Principles and Experimental Design
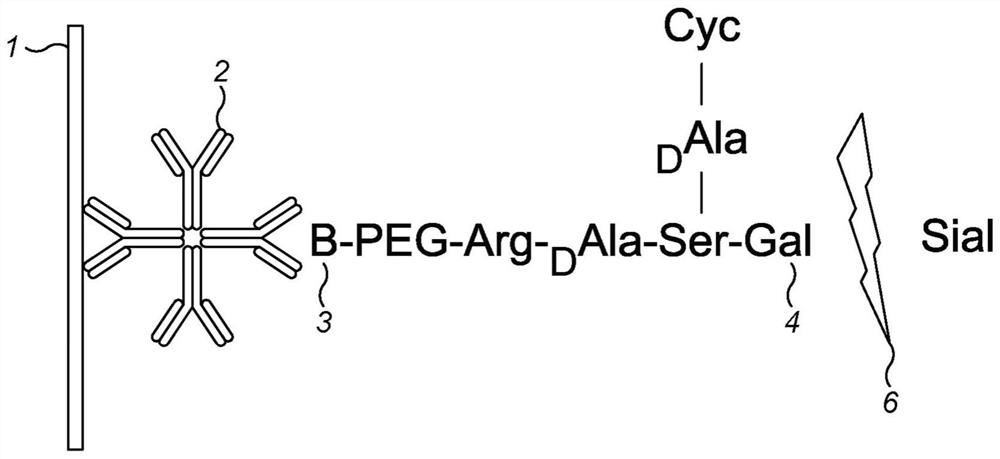
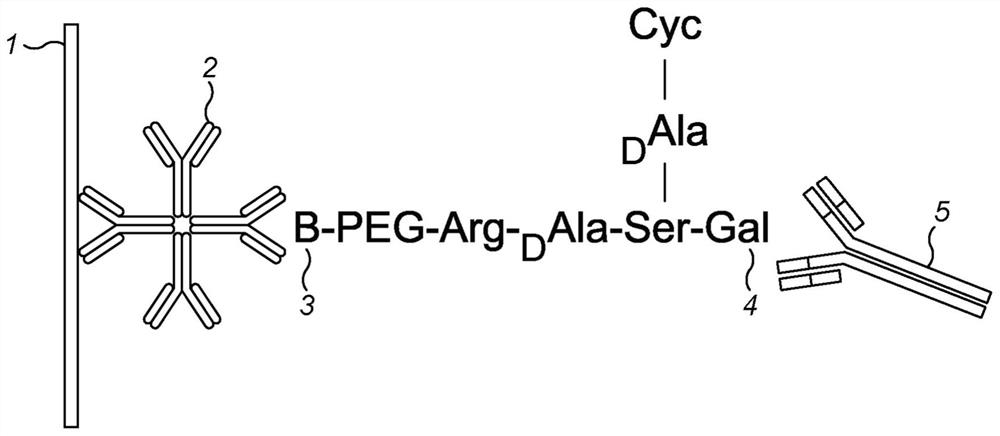
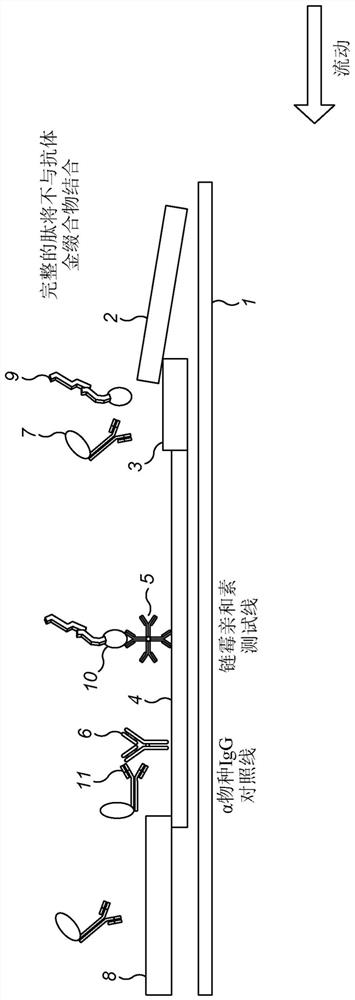
[0331] In summary, the principle works on the basis that antibodies recognize the chemical product of a sialidase reaction, where the chemical substrate is a peptide specifically designed to react with the sialidase, and that the reaction product is then recognized by an antibody raised against said synthetic product. As shown in Figures 1 and 2, glycopeptides containing sialic acid and having a biotin tag are provided. When contacted with a test sample containing sialidase activity, the sialylated group is cleaved from the glycopeptide by the sialidase to expose the pendant galactosyl group on the peptide. Antibodies raised against the desialylated product then bind specifically to the cleaved product. By using an antibody-gold conjugate and a lateral flow strip with a streptavidin test line, the presence of desialylated product can be detected as a red line, as well as sialidase activity measured directl...
example 2
[0333] Example 2: Peptide Design
[0334] In the design of candidate peptide sequences, many factors are considered:
[0335] size
[0336] In general, molecules with a molecular weight (MW) of less than 5000 Daltons are less likely to stimulate a good immune response in the host organism. The peptides were planned as relatively short sequences designed to be conjugated to KLH (keyhole limpet hemocyanin), resulting in more and more potent immunogens. A range of different lengths (9-20 amino acids) is proposed to cover any potential performance variation.
[0337] conjugation chemistry
[0338] Two different conjugation chemistries were used. For two of the peptides, a cysteine tag was incorporated into the structure, allowing it to be conjugated to a carrier protein using standard maleimide-based chemistry. For the other three peptides, a relatively new hydrazine-based chemistry was used as a more controllable process than cysteine chemistry because there is less ...
example 3
[0346] Example 3: Peptide Synthesis
[0347] Galactose-tagged peptides were synthesized using solid-phase chemistry on an automated microwave synthesizer. All peptides were purified using reverse phase HPLC and characterized by electrospray LCMS. To label the galactose moiety with sialic acid, an enzymatic approach using transsialic acid (TcTS) from T. cruzi and fetuin as sialic acid donor was employed. Figure 7 and 8 The synthetic methods used are summarized.
[0348] and related peptide immunogens conjugated to carrier proteins, two biotinylated derivatives of each sequence, one of which was labeled with sialic acid, were also synthesized. These biotinylated derivatives will form the basis for a lateral flow assay format. Figure 9 shows the peptides synthesized for this work based on various immunizations. Peptides labeled with sialic acid are designated with a "c"; eg, MOL136c. Peptides lacking a sialylation group do not have this designation (eg, MOL136).
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com