Crystal form of rosustat and preparation method thereof
A technology of roxadustat and crystal form, which is applied in the directions of organic chemical methods, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of poor solubility, insolubility, and low solubility of crystal form A, and achieves the preparation method. Simple, high crystal form purity, good crystal form stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Embodiment 1: Preparation of crystal form D
[0225] Mix 1g of roxadustat sample with 10mL of acetone / water=3:1 mixed solvent, heat to 55°C until the sample is completely dissolved, slowly cool down to 50°C, add 20ml of ethanol, add 20mg of seed crystals, keep stirring for 6h, filter , the sample was vacuum-dried at 50°C for 24 hours to obtain 0.91 g of Form D with a purity of 99.66%.
Embodiment 2
[0226] Embodiment 2: the preparation of crystal form D
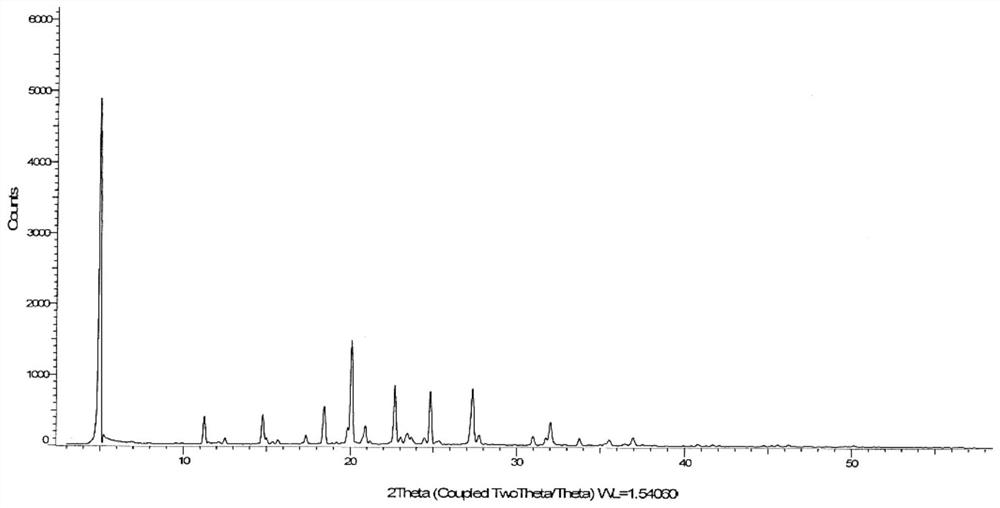
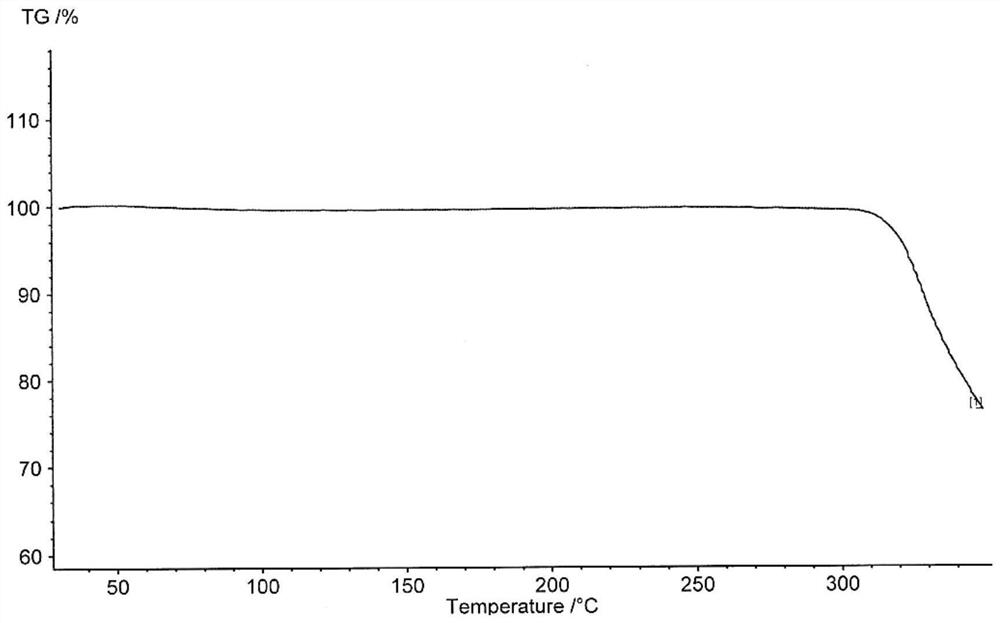
[0227] Mix 1g of roxadustat sample with 10mL of acetone / water=4:1 mixed solvent, heat to 65°C until the sample is completely dissolved, slowly cool down to 50°C, add 30ml of ethanol, add 10mg of seed crystals, keep stirring for 6h, filter , the sample was vacuum-dried at 50°C for 24h to obtain 0.95g of Form D with a purity of 99.68%. Its X-ray diffraction pattern is as figure 1 shown. Its TGA spectrum is as figure 2 shown.
Embodiment 3
[0228] Embodiment 3: the preparation of crystal form D
[0229] Mix 1g of roxadustat sample with 10mL of acetone / water=3:1 mixed solvent, heat to 55°C until the sample is completely dissolved, slowly cool down to 35°C, add 30mg of seed crystals, keep stirring for 6h, and slowly cool down to 2°C , and filtered to obtain 0.93g of Form D with a purity of 99.65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com