Marker for evaluating responsiveness and prognosis survival of advanced bladder cancer anti-tumor immunotherapy and application of marker
An anti-tumor immune and reactive technology, applied in the determination/examination of microorganisms, biochemical equipment and methods, etc., can solve unreported problems, achieve less pain for patients, improve sensitivity and accuracy, and reduce treatment costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Model Construction and Effect Verification
[0028] 1. Subjects
[0029] The research subjects in this example were selected from patients with advanced bladder cancer who received anti-PD-1 / PD-L1 therapy in the IMvigor 210 trial. The clinicopathological data and gene expression processing data of these patients came from a development data resource based on the R environment—— IMvigor210CoreBiologies. The inclusion and exclusion criteria of patients are as follows:
[0030] (1) Patients with advanced bladder cancer who are ineffective to platinum-based chemotherapy and receive tumor immunotherapy;
[0031] (2) Complete curative effect information and clinical follow-up data;
[0032] (3) have whole-transcriptome RNA sequencing data;
[0033] (4) Patients with unknown tumor immunotherapy results or incomplete survival data were excluded.
[0034] 2. Experimental process
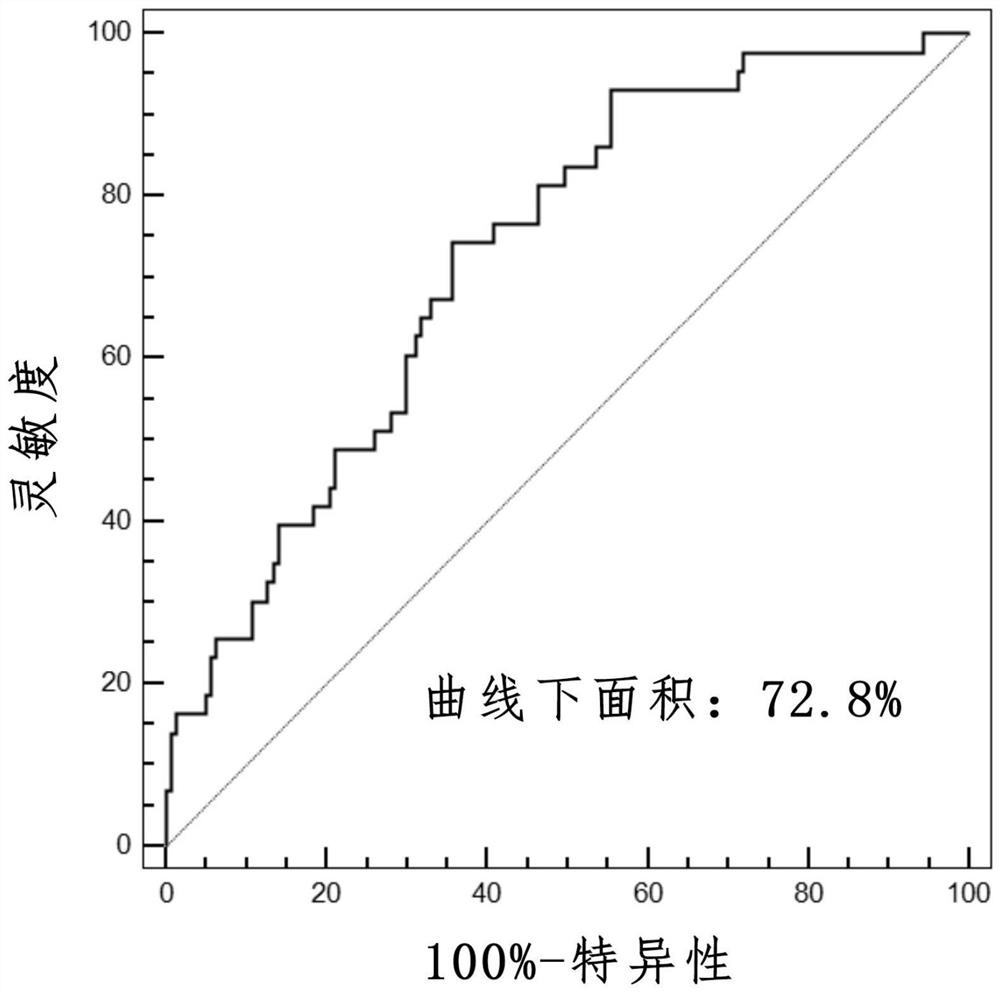
[0035] A total of 298 patients who met the above criteria were included in the stud...
Embodiment 2
[0044] Embodiment 2 kit (1)
[0045] Including detection reagents and instructions, the detection reagents are composed of reagents for detecting the expression of the following six genes: CDH18, CXCL10, FOXN4, SLC6A4, CXCL9 and PCDH11X;
[0046] The instruction manual records the following:
[0047] Nomogram score = 316.215877454-(4.073676019×CDH18)-(7.692307692×CXCL10)-(5.176616243×FOXN4)-(2.733887894×SLC6A4)-(2.520670945×CXCL9)-(3.8812067)PC;
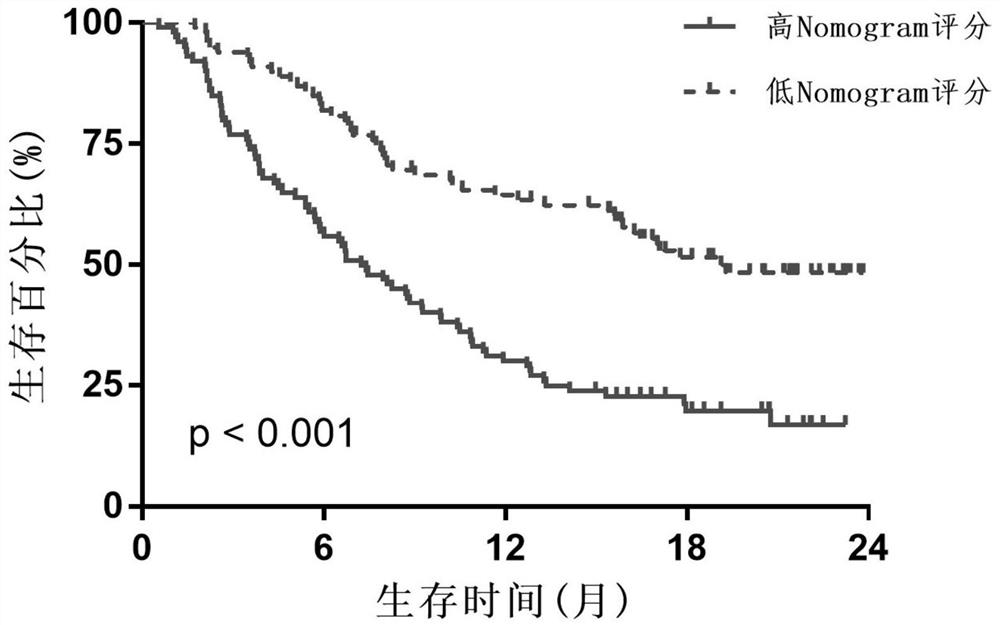
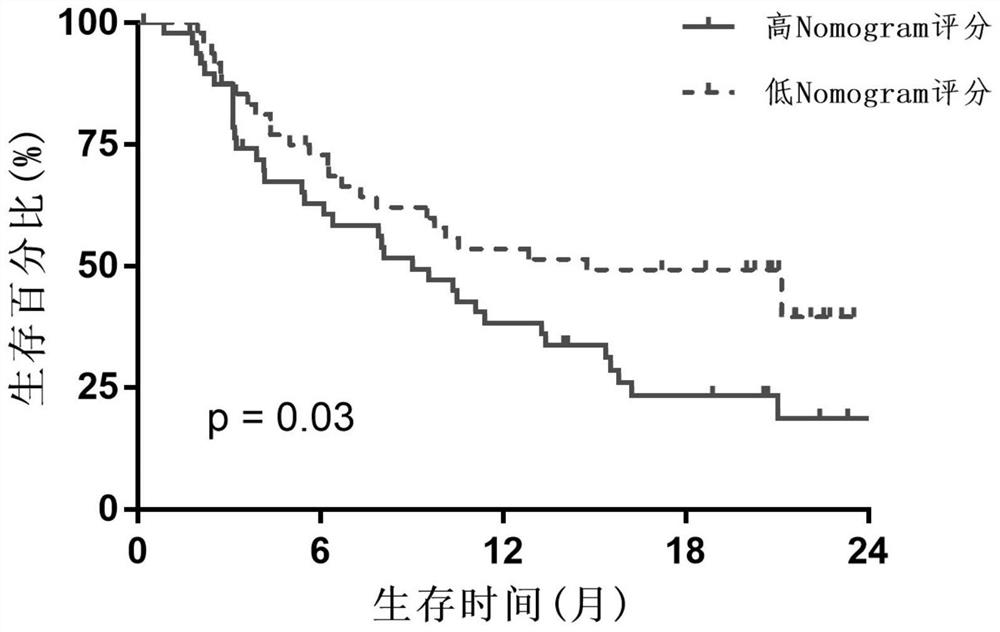
[0048] If the Nomogram score is less than 183, it means predicting immunotherapy response and prognosis survival is a low-risk group, and its 3-year survival rate is 45.1%-52.0%; if the Nomogram score is greater than 183, it means predicting immunotherapy responsiveness The prognosis and survival are high-risk group, and its 3-year survival rate is 20.0%-29.8%.
Embodiment 3-8
[0049] Embodiment 3-8 kit (two-seven)
[0050] Kit 2 Kit 3 Kit 4 Kit 5 Kit 6 Kit 7 Reagents for detection of CDH18 expression √ Reagents for detecting the expression of CXCL10 √ Reagents for detecting the expression of FOXN4 √ Reagents for detecting the expression level of SLC6A4 √ Reagents for detecting expression of CXCL9 √ Reagents for detecting the expression level of PCDH11X √
[0051] All the above kits also include instructions, and the instructions record the following content: when the gene expression level is higher than the normal value of a single gene expression level, it belongs to the low-risk group, and the treatment response is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com