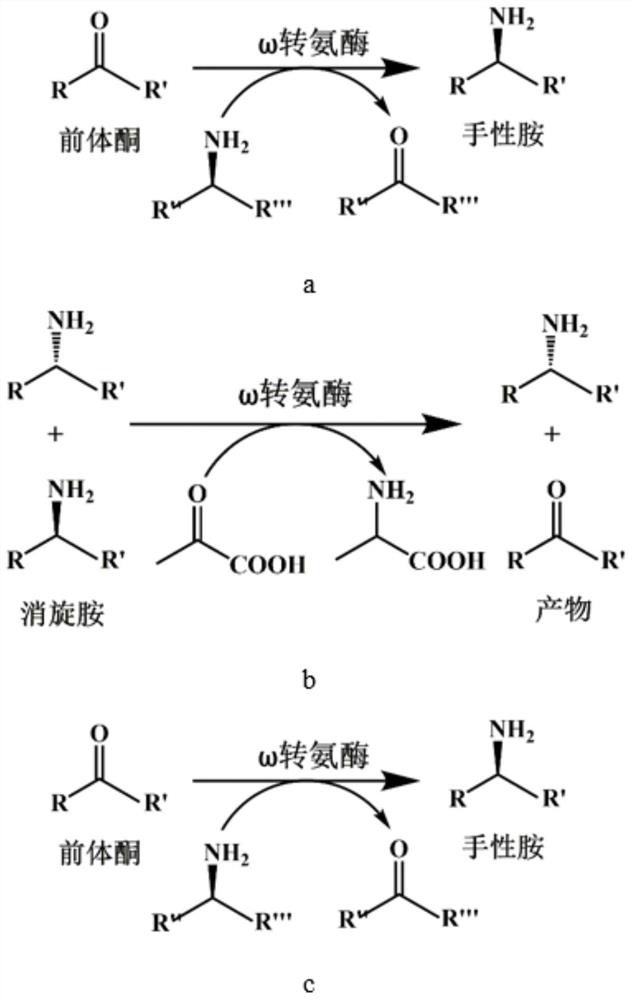
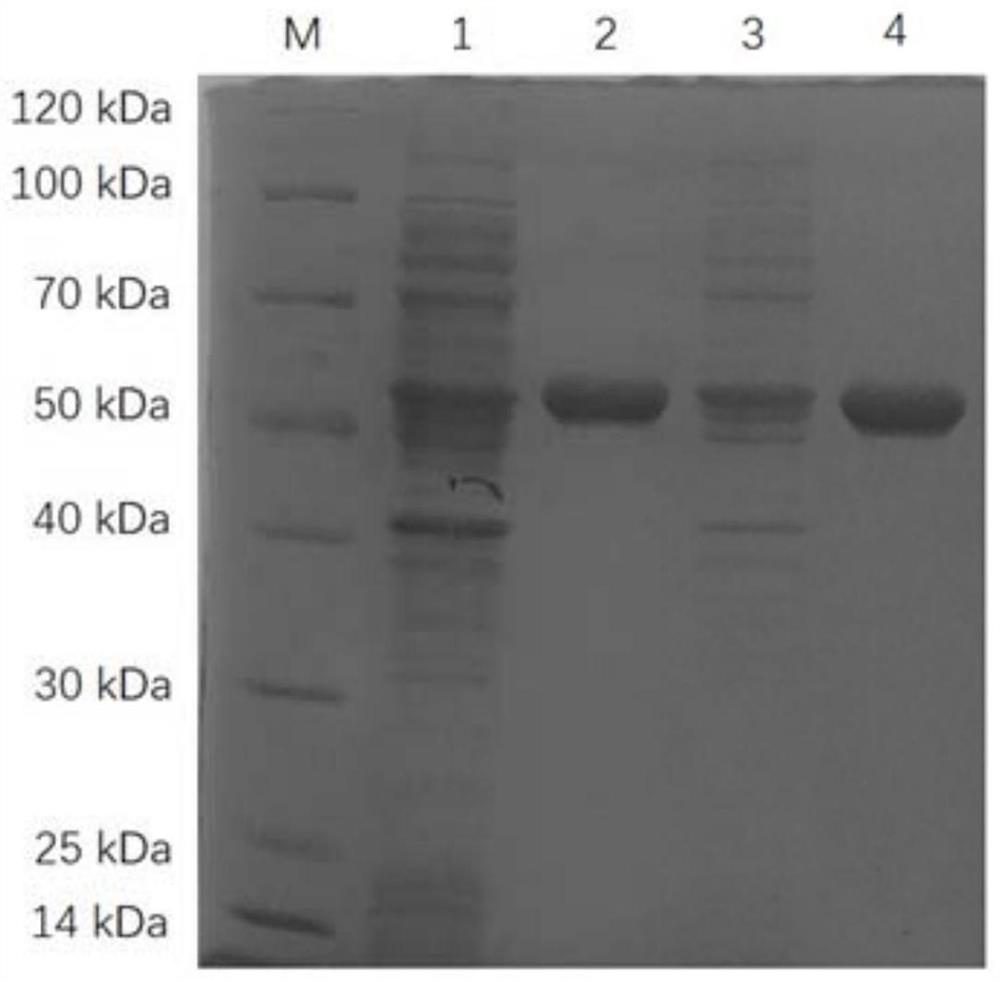
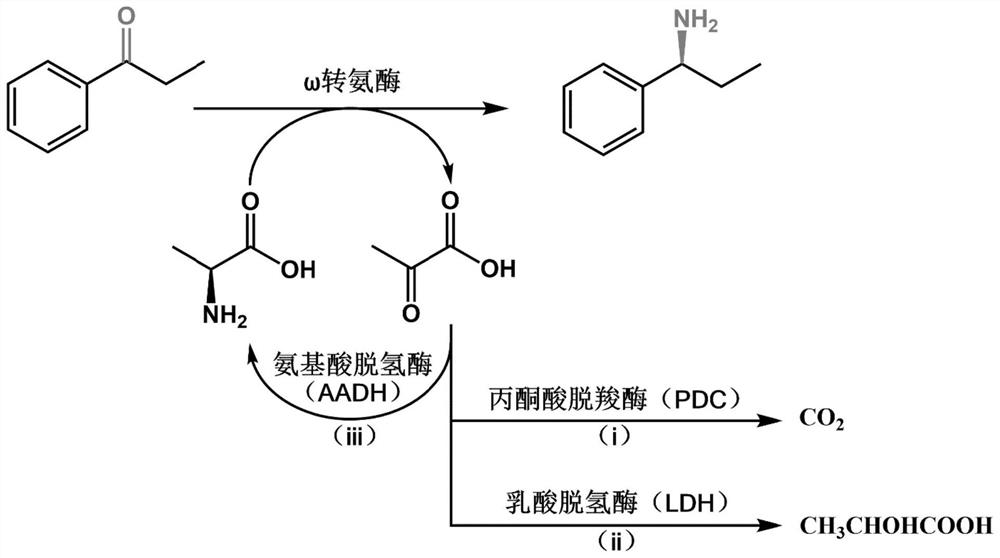
Omega transaminase as well as mutant, recombinant plasmid, genetically engineered bacterium and application thereof
A technology of genetically engineered bacteria and recombinant plasmids, applied in the fields of genetic engineering, application, transferase, etc., can solve the problems of low conversion efficiency, low catalytic activity of 1-propiophenone, low conversion rate, etc., and achieve excellent catalytic activity, reaction Mild conditions and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Amplification of the omega transaminase gene bpta derived from Burkholderia tumoriformis
[0046] The ω transaminase gene bpta of the present invention is derived from Burkholderia phymatum STM815, the ω transaminase BPTA of the present invention belongs to type I PLP-dependent enzyme, and the nucleotide of the ω transaminase gene bpta encoding the ω transaminase BPTA The sequence is shown as SEQ ID No: 1, and the size is 1437bp.
[0047] Using the Shanghai Sangon Biotech Gram-negative Bacteria Genomic DNA Mini-Prep Kit, and in strict accordance with the instructions on the operation of Burkholderia tumoriform DNA samples were purified to obtain Burkholderia tumoriformum (Burkholderiaphymatum STM815 ) whole genome.
[0048] According to the sequence analysis results, the primers for amplifying the omega transaminase gene bpta were designed:
[0049] bptaF:5'-ggaattccatatgagtttcaggacagaagaaatcgc-3'
[0050] bptaR:5'-cccaagcttacgcaagcccgagttgttgt-3'
[0051...
Embodiment 2
[0053] Example 2: BPTA W82A , BPTA W82A / M78F , BPTA W82A / I284F , BPTA W82A / T440Q , BPTA M78F / W82A / I284F / T440Q Mutant strain construction
[0054] The recombinant plasmid containing the mutant ω transaminase gene bpta-M78F / W82A / I284F / T440Q used in the present invention is constructed in an iterative manner: first, the recombinant plasmid bpta-pET-28a is used as a template, and the following primer pair ① As a primer, the recombinant plasmid bpta-W82A-pET-28a was obtained; then, the recombinant plasmid bpta-W82A-pET-28a was used as a template, and the following primer pair ② was used as a primer to obtain the recombinant plasmid bpta-W82A / M78F-pET-28a; Then use the recombinant plasmid bpta-W82A / M78F-pET-28a as a template, and the following primer pair ③ as primers to obtain the recombinant plasmid bpta-M78F / W82A / I284F-pET-28a; finally use the recombinant plasmid bpta-M78F / W82A / I284F -pET-28a was used as a template, and the following primer pair ④ was used as a primer to obt...
Embodiment 3
[0066] Embodiment 3: Contain the construction of the recombinant genetic engineering strain of transaminase gene bpta and mutant gene thereof
[0067] Escherichia coli BL21 (DE3) was inoculated in LB medium, and BL21 competent cells were prepared according to conventional methods in the art.
[0068] Transfer the recombinant plasmid obtained in the above-mentioned Example 2 into E. coli BL21 competent cells, evenly spread it on a kanamycin-resistant plate, culture it upside down at 37°C for 16 hours, pick a single colony and verify that it is a positive clone, and pass through LB medium Cultivate and obtain recombinant genetically engineered strains.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com