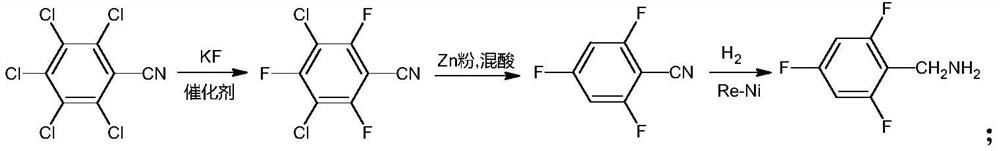
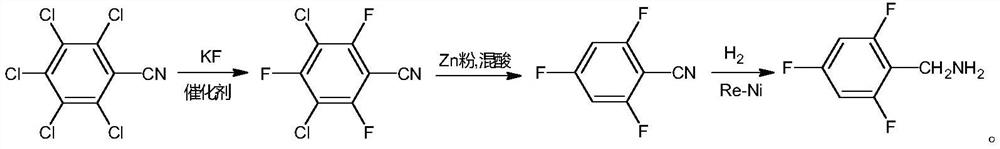
Preparation method of compound 2,4,6-trifluorobenzylamine
A technology of trifluorobenzylamine and compounds, which is applied in the preparation of organic compounds, the preparation of amino compounds, chemical instruments and methods, etc., can solve the problems of low yield, difficult recovery and application, and many hydrogenation reaction by-products, etc. The effect of high yield, low cost of raw materials and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In a 2000ml three-necked flask, add 200g of pentachlorobenzonitrile (0.726mol), 160g of anhydrous potassium fluoride (2.75mol), and 1000g of sulfolane, stir, and under nitrogen protection, add 6.0g of 18-crown-6 and heat up to 125 ℃-135℃ reflux reaction for 4h. After the reaction is complete, lower the temperature to below 50°C, filter, add 2000ml of water and 800ml of cyclohexane to the filtrate, stir, separate layers, take the organic layer and wash it with 400ml water, separate layers, dry with anhydrous sodium sulfate, filter, and evaporate the filtrate under reduced pressure. The solvent was removed to obtain 154.8 g of 3,5-dichloro-2,4,6-trifluorobenzonitrile (yield 94.3%, HPLC purity 98.7%).
Embodiment 2
[0034] Add 20g of 3,5-dichloro-2,4,6-trifluorobenzonitrile (0.0885mol), 300g of water, 23.2g of zinc powder (0.355mol) into a 500ml three-necked flask, stir, raise the temperature to 90°C, and add dropwise 43.1 A mixture of 30% concentrated hydrochloric acid (0.355mol) and 80g acetic acid (1.332mol) (mixed acid molar ratio 1:3.8), added dropwise for 2 hours, then kept at 95°C-105°C for 4 hours, and cooled down to room temperature after the reaction was complete. Add 250ml of dichloromethane to stir, filter, the filtrate is layered, take the organic layer and adjust the pH to 7 with saturated aqueous sodium bicarbonate solution, then separate the layers, wash the organic layer with 150ml of water, dry over anhydrous magnesium sulfate, filter, and distill the filtrate under reduced pressure 11.0 g of 2,4,6-trifluorobenzonitrile was obtained (yield 79.1%, GC purity 97.2%).
Embodiment 3
[0036] Add 20g of 3,5-dichloro-2,4,6-trifluorobenzonitrile (0.0885mol), 300g of water, 23.2g of zinc powder (0.355mol) into a 500ml three-necked flask, stir, raise the temperature to 90°C, and add dropwise 43.1 A mixture of 30% concentrated hydrochloric acid (0.355mol) and 60g acetic acid (0.999mol) (mixed acid molar ratio 1:2.8), added dropwise for 2 hours, then kept at 95°C-105°C for 5 hours, and cooled down to room temperature after the reaction was complete. Add 250ml of dichloromethane and stir, filter, the filtrate is layered, take the organic layer and adjust the pH to 7 with saturated aqueous sodium bicarbonate solution, then separate the layers, wash the organic layer with 150ml of water, dry over anhydrous magnesium sulfate, filter, and distill the filtrate under reduced pressure 10.7 g of 2,4,6-trifluorobenzonitrile was obtained (yield 77.0%, GC purity 97.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com