A kind of cytochrome p450 epoxidase and its application
A technology of cyclooxygenase and epoxy, which is applied in the field of bioengineering to achieve the effects of speeding up the industrialization process, high catalytic specificity, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
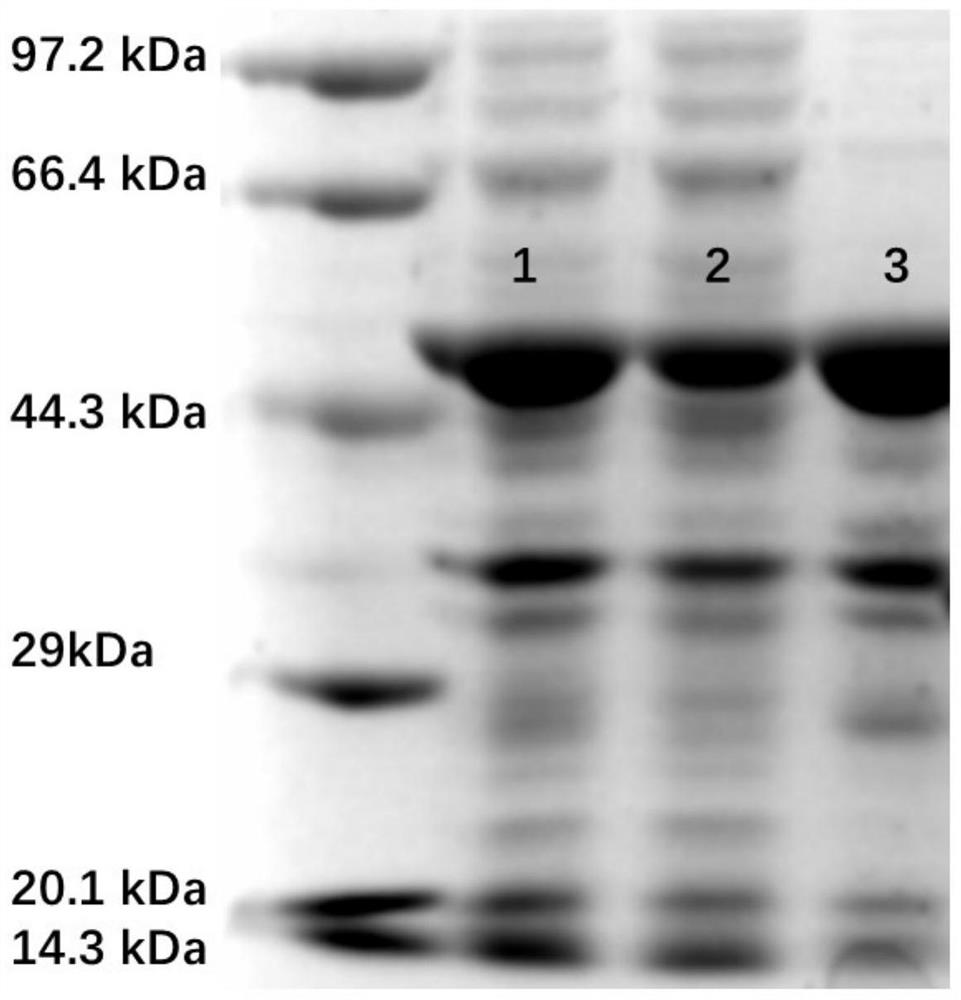
[0031] Example 1: Expression and purification of CytP enzyme
[0032] Construction of genetically engineered bacteria and protein expression:
[0033] Take the nucleotide sequence (SEQ ID NO. 2) of the target protein-coding gene in Pseudomonas putida KT2440 as the template, and use F1 and R1 as primers (underlined are BamH I and EcoR I restriction enzymes respectively). site) PCR amplification, amplification conditions are:
[0034] 95°C for 5min, 29 cycles (98°C for 10s, 55°C for 15s, 72°C for 1.5min), 72°C for 5min.
[0035] F1: caaatgggtcgcggatccATGGAGATCC (SEQ ID NO. 3);
[0036] R1: tcgacggagctcgaattcTTACCAAATCAC (SEQ ID NO. 4).
[0037] The cDNA sequence of the CytP gene coding region was obtained. After the PCR product was recovered, it was connected with the pET-28a(+) plasmid vector that had been digested with the same double enzyme through homologous recombination to obtain the recombinant expression plasmid pET-28a(+)-p28p. The recombinant plasmid pET -28a(+)-p2...
Embodiment 2
[0040] Example 2: CytP enzyme activity verification
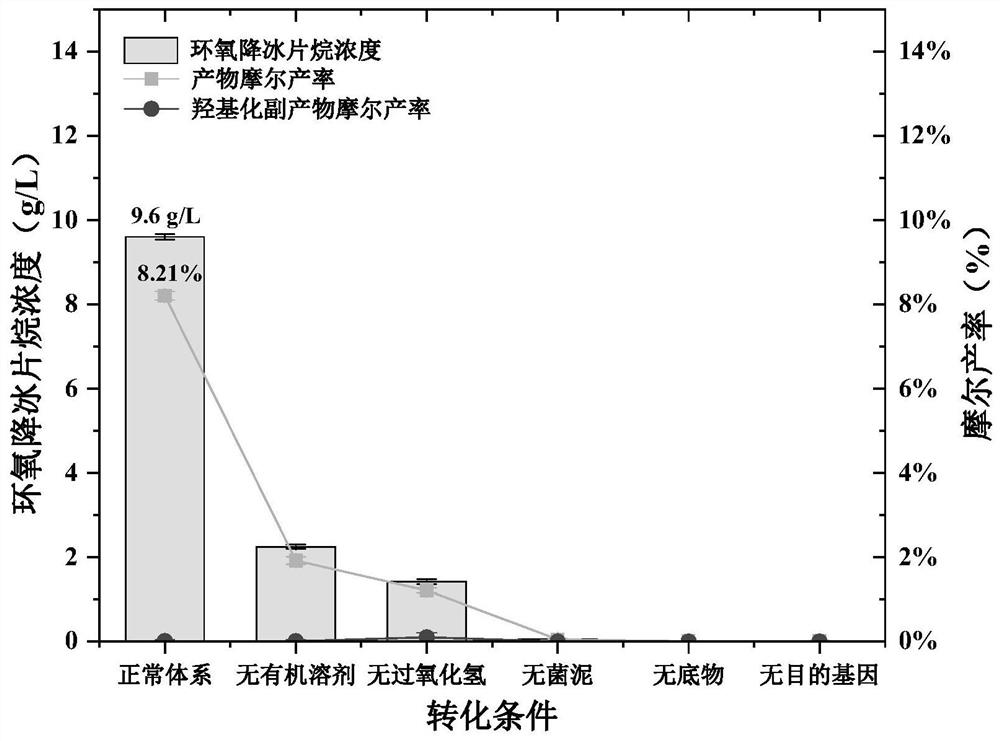
[0041]The strains stored in the glycerol tube were spread on LB solid medium, and incubated at a constant temperature of 37°C until a single clone was grown. Pick a single clone into a fresh LB liquid medium, and cultured at a constant temperature of 200rpm and 37°C. After 12 h, the activation solution was obtained, and the activation solution was inoculated into fresh TB medium in an amount of 1 mL / 100 mL. After culturing for 2 h, IPTG with a final concentration of 0.2 mM was added, and the culture was induced at 25 °C for 14 h, and the cells were collected after the end.
[0042] 0.2 g of whole cells expressing CytP protein after induction culture, 1 g of norbornene (C 7 H 10 O, NBE), 320 μL of 30% hydrogen peroxide, 2.18 mL of phosphate buffer (100 mmol / L KCL, pH 7.4) and 7.5 mL of ethyl acetate, centrifuged at 5000 r / min for 10 min after reaction at 25°C for 48 h, and sucked the upper organic phase, It was dried over ...
Embodiment 3
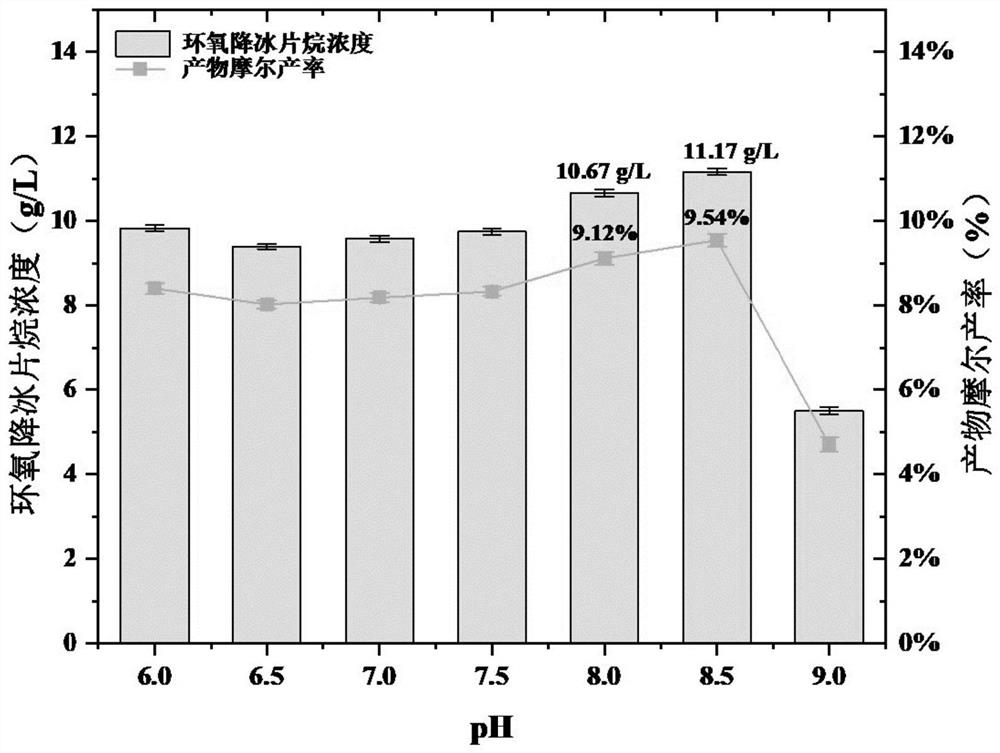
[0047] Example 3: Whole Cell Optimum Response pH
[0048] For specific embodiments, see Example 2, the difference is that 0.2 g of whole cells were prepared in phosphate buffer solutions of pH6.0, pH6.5, pH7.0, pH7.5, pH8.0, pH8.5 and pH9.0, respectively. 10 mL of a two-phase transformation system (organic phase: aqueous phase = 3:1) was transformed for 48 h, the yield of norbornane was measured according to the above detection method, and the molar yield of epoxidation was calculated. The results showed that the epoxidation activity of CytP enzyme increased with the increase of pH in pH 6.0-pH 8.5, reaching 10.67 g / L at pH 8.0, and reaching a peak around pH 8.5. The yield was 11.17 g / L with a molar yield of 9.54%, followed by a decrease in epoxidation activity with further increase in pH. This indicates that higher pH (alkaline environment) is more favorable for CytP to catalyze epoxidation reaction, and whole cells have better epoxidation activity at pH 8.0-8.5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com