Continuous flow synthesis process of tert-amyl hydroperoxide
A technology of tert-amyl hydroperoxide and hydrogen peroxide is applied in the preparation of peroxy compounds, the preparation of organic compounds, organic chemistry and other directions, and can solve the problems of increasing waste water, increasing the difficulty of post-treatment, and long total reaction time, etc. Achieve the effect of reducing the production of by-products, shortening the total reaction time, and optimizing the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
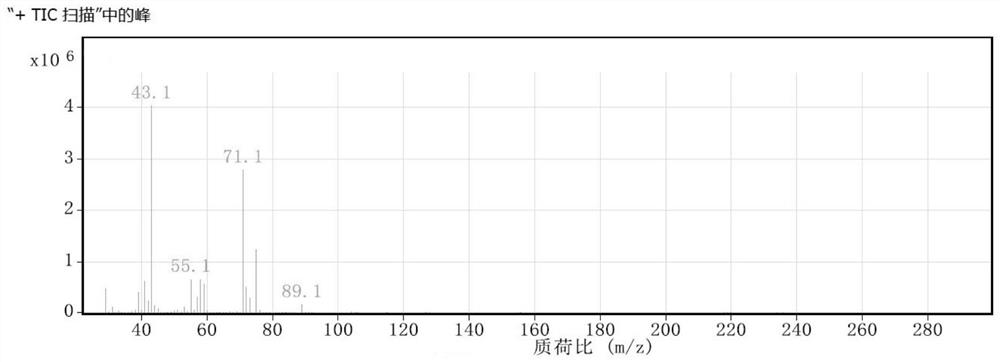
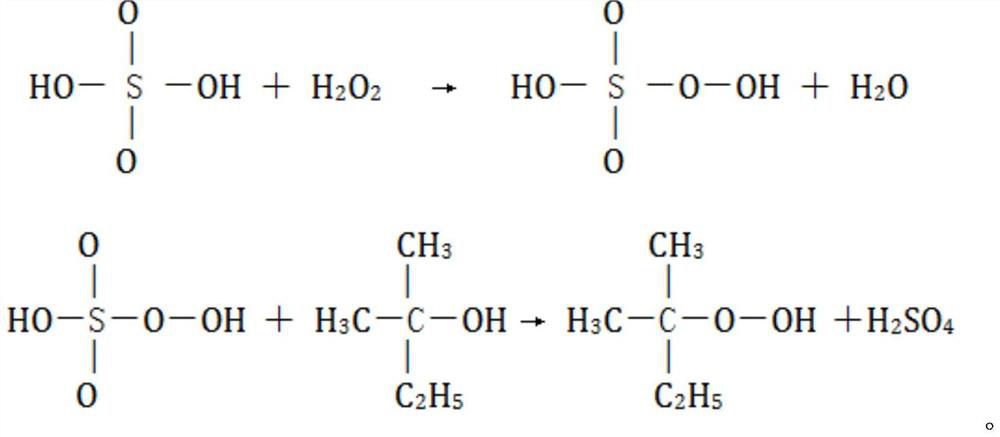
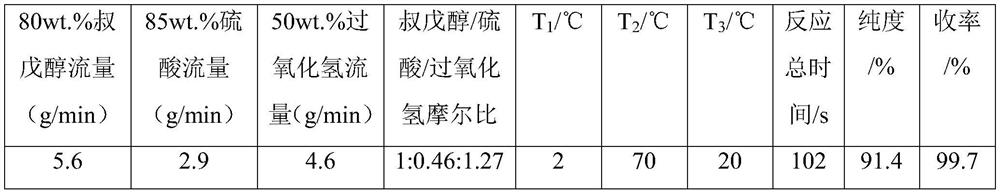
[0033] The integrated continuous flow reactor adopts a unitized structure, which includes a premixing unit, an esterification unit and a quenching unit in sequence. Use a constant flow pump to transport sulfuric acid and hydrogen peroxide to the premixing unit for reaction to generate peroxymonosulfuric acid; then use a constant flow pump to transport tert-amyl alcohol to the esterification unit. Alcohol reacts to obtain a reaction solution; the reaction solution further flows into the quenching unit to quench the reaction, collect the reaction mother liquor, and separate layers to obtain tert-amyl hydroperoxide, its mass spectrum is as follows figure 1 , the peak data of tert-amyl hydroperoxide in the figure are shown in Table 8. The consumption of raw material, the reaction temperature of each unit, product purity and yield are shown in Table 1, wherein T 1 , T 2 , T 3 represent the temperature of premix unit, esterification unit and quenching unit respectively.
[0034]...
Embodiment 2
[0037] Reaction process is with embodiment 1, and the consumption of raw material, the reaction temperature of each unit, product purity and yield are shown in Table 2, wherein T 1 , T 2 , T 3 represent the temperature of premix unit, esterification unit and quenching unit respectively.
[0038] Table 2 The amount of raw materials, the reaction temperature of each unit, product purity and yield data table
[0039]
Embodiment 3
[0041] Reaction process is with embodiment 1, and the consumption of raw material, the reaction temperature of each unit, product purity and yield are shown in Table 3, wherein T 1 , T 2 , T 3 represent the temperature of premix unit, esterification unit and quenching unit respectively.
[0042] Table 3 The amount of raw materials, the reaction temperature of each unit, product purity and yield data table
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com