A mutant based on autophagy key protein atg7 and its application
A key protein and mutant technology, applied in the field of biotechnology and anti-aging treatment, to achieve the effects of controllable purity, improved cell proliferation status, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 is based on the preparation of a mutant of the key autophagy protein ATG7.
[0024] A mutant based on the key autophagy protein ATG7, the amino acid sequence of which is SEQ ID No1, wherein the 411st and 413th lysines are mutated to glutamine, and the 513th, 514th and 516th amino acids are mutated to arginine.
[0025] The preparation method of the mutant based on the key autophagy protein ATG7 is as follows: using a eukaryotic expression system, overexpressing the plasmid of the mutant ATG7 with a flag tag in HEK293T cells, collecting the cells after 48 hours of expression, extracting the protein, Add flag-beads to the protein extract, mix overnight at 4°C, collect flag-beads at 4°C, use flag-peptide to compete for the elution of the ATG7 protein mutant, and the eluent is the purified ATG7 protein mutant.
Embodiment 2
[0026] Example 2 is based on the pharmacological study of mutants of the key autophagy protein ATG7.
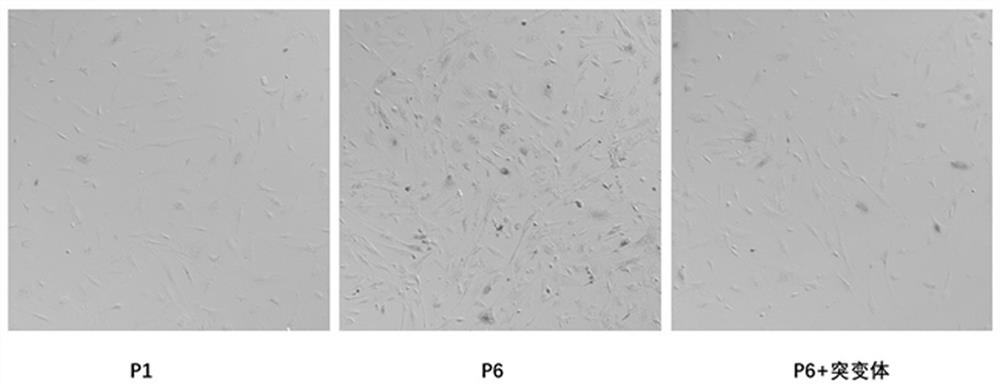
[0027] 1. Establishment of senescent cell model and experimental grouping.
[0028] Firstly, primary mouse embryonic fibroblasts (MEFs) were cultured. Take 13.5-day-old mouse embryos, separate the trunk into 1×PBS; discard the PBS, add 0.125% trypsin, and digest in a 37°C incubator for 15-20min. Planting medium (DMEM+15% serum) was used to stop digestion, centrifuged at room temperature, and the precipitate was resuspended and planted in a 6cm2 cell culture dish. After genotype identification, ATG7- / - MEFs were used to infect lentivirus negative control and flag-ATG7 mutants, respectively.
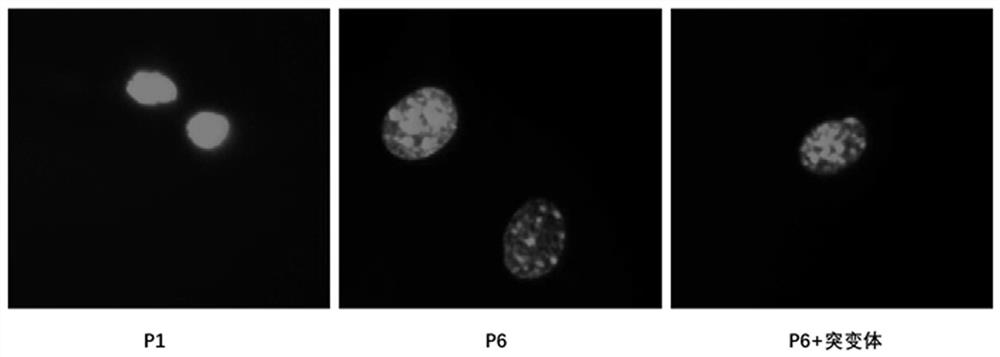
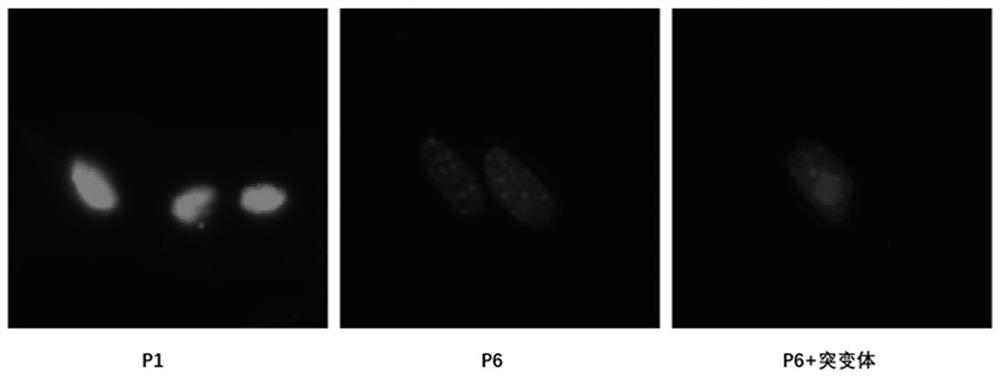
[0029] 2. Detection of cell nucleus morphology.
[0030] MEF cells stably overexpressing the negative control and flag-ATG7 mutants were subcultured in vitro, and poly-lysine was coated with cell slides in a 12-well plate at room temperature for 2 hours. The first-generation MEFs pass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com