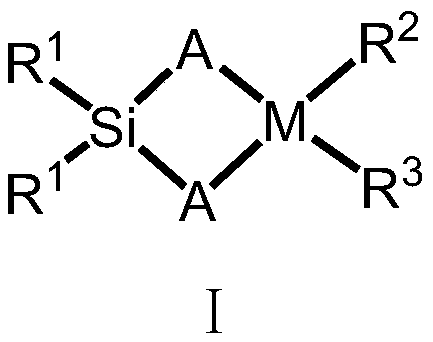
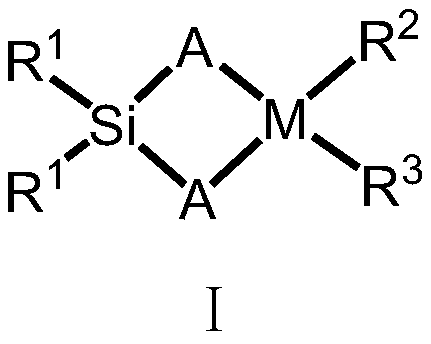
Silicon-bridged metallocene complex with C2 symmetrical structure and application thereof
A technology of metallocene complexes and symmetrical structures, which is applied in the direction of silicon organic compounds, compounds of group 4/14 elements of the periodic table, titanium organic compounds, etc., can solve the problem of low isotacticity of polypropylene, and achieve simple preparation, High activity and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
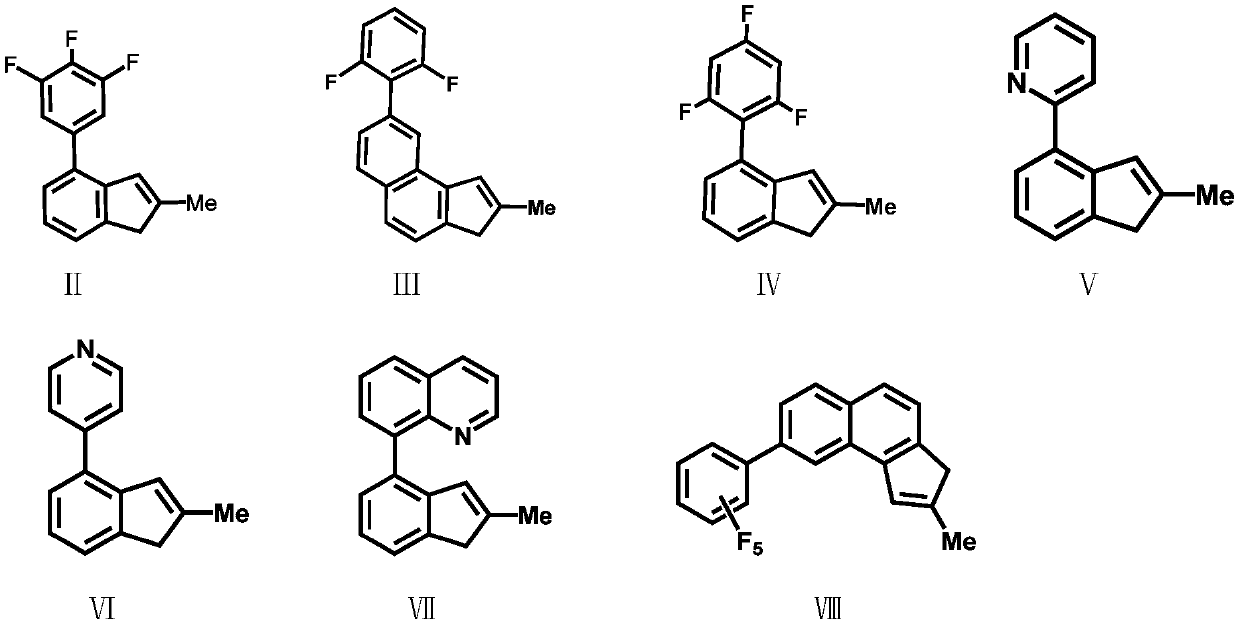
[0038] Compound Formula VII: Preparation of 2-methyl-4-8-quinoline-indene
[0039] 8-Bromoquinoline (2.08g / 10mmol) was dissolved in 50mL of anhydrous tetrahydrofuran, and n-butyllithium (2.5M, 4mL / 10mmol) was added dropwise at -78°C, gradually raised to room temperature, and stirred at room temperature for 16 hour, trimethyl borate (1.04g / 10mmol) was added dropwise at -78°C, gradually warmed to room temperature, stirred at room temperature for 16 hours, water was gradually added at 0°C, and then 3.4mL of 3M aqueous hydrochloric acid was added dropwise. Extract with ether, adjust the pH to neutral with sodium bicarbonate, and recrystallize from acetone and n-hexane to obtain intermediate product 1 (1.5 g, yield 87%). Intermediate product 1 structural formula:
[0040]
[0041] The obtained intermediate product 1 (1.72g / 10mmmol), 2-methyl-4-bromoindanone (1.46g / 10mmol), 0.005mol% Pd(OAc) 2 Dichloromethane solution, tetrabutylammonium bromide (3.22g / 10mmol), potassium carbon...
Embodiment 2
[0046] Preparation of Ligand L1 Bis(2-methyl-4-3,4,5-trifluorophenyl-indene)dimethylsilyl
[0047] Under a nitrogen atmosphere, the compound formula II (2.6g / 10mmol) was dissolved in 50mL of anhydrous tetrahydrofuran, and n-butyllithium (2.5M, 4mL / 10mmol) was added dropwise at -78°C, and gradually increased under nitrogen protection. Return to room temperature, stir at room temperature for 16 hours, then add the lithium salt solution formed by the reaction into a tetrahydrofuran solution of dimethyldichlorosilane (5 mmol) at -78°C, gradually rise to room temperature and continue stirring for 16 hours. Remove the solvent under reduced pressure, add dry toluene or n-hexane to wash three times, and filter. Ligand L1 was obtained by removing the solvent. The ligand L1 was 5.13g, 8.92mmol, yield: 89%. Anal. Calcd for C 34 h 26 f 6 Si: C, 70.82; H, 4.54. Found: C, 68.83; H, 4.94.
Embodiment 3
[0049] Preparation of Ligand L2 Bis(2-methyl-4-2,6-difluorophenyl-indene)dimethylsilyl
[0050] The experimental procedure was the same as in Example 2, and 4.54 g, 8.41 mmol of ligand L2 was obtained, yield: 84%. Anal. Calcd for C 34 h 28 f 4 Si: C, 75.53; H, 5.22. Found: C, 73.02; H, 5.67.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com