Hypersensitive microRNA electrochemical detection method of incision enzyme driven multi-legged DNA molecular machine
A DNA molecule and molecular machine technology, applied in the field of ultrasensitive microRNA electrochemical detection, to achieve the effect of improving the detection and analysis performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
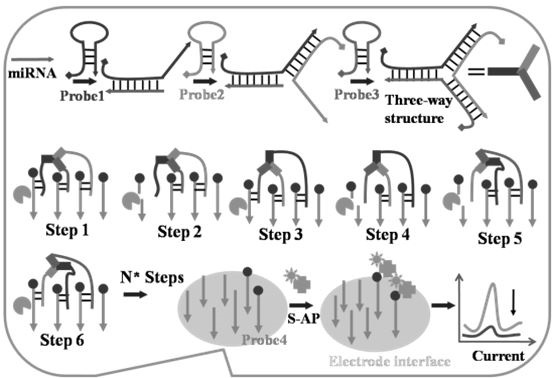
[0044] Such as figure 1 It is a design diagram of the electrochemical sensing detection principle based on the three-fork walking DNA molecular machine in Example 1 of the present invention.
[0045] (1) The biological probe sequences used in the electrochemical detection process of microRNA in this embodiment are shown in Table 1:
[0046] Table 1: Biological probe sequences used in the electrochemical detection of microRNA
[0047]
[0048] (2) Biological probe preparation Gold electrode interface preparation:
[0049] The gold electrode was first scanned in 0.5M dilute sulfuric acid for 12 weeks, and then polished on the buckskin with 0.3 μM and 0.05 μM aluminum powder for 10 min, the surface of the gold electrode was washed with water, and then the surface of the gold electrode was washed with piranha solution (H 2 SO 4 :H 2 o 2 =3:1) for 3 times, 5 min each time, then, wash the surface of the gold electrode with water, and dry the surface of the gold electrode wit...
Embodiment 2
[0055] (1) Feasibility verification results of nucleic acid testing:
[0056] Agarose gel (3%) electrophoresis verification polypod DNA nanostructure construction, the results are as follows figure 2 Shown: Lane 1 is a 500bp DNA marker; Lanes 2, 3, 4, and 5 are target microRNA and detection probes (probe1, 2, 3); Lane 6 is the assembled chain microRNA+probe1; Lane 7 is the assembled chain microRNA+probe1 +probe2; lane 8 is the assembled chain microRNA+probe1+probe2+probe3; lane 9 is probe1+probe2+probe, the maker lane is 500bp DNA ladder (Takara), lanes 1 to 4 are assembled chains, as an electrophoresis control, lanes 5 to 7 is the DNA assembly process. Compared with the control band, the band is gradually reared. This is because the assembled chain is gradually assembled into a large-molecular-weight DNA hybrid, which leads to a slowdown in the electrophoretic movement rate. The result suggests that the polypod DNA nanostructure The construction is feasible, and the respons...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com