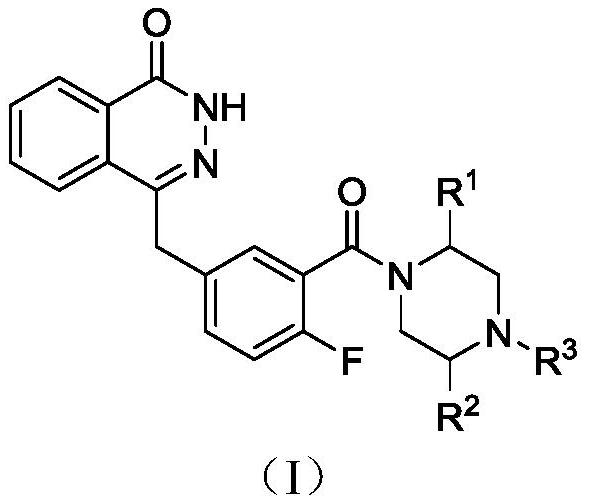
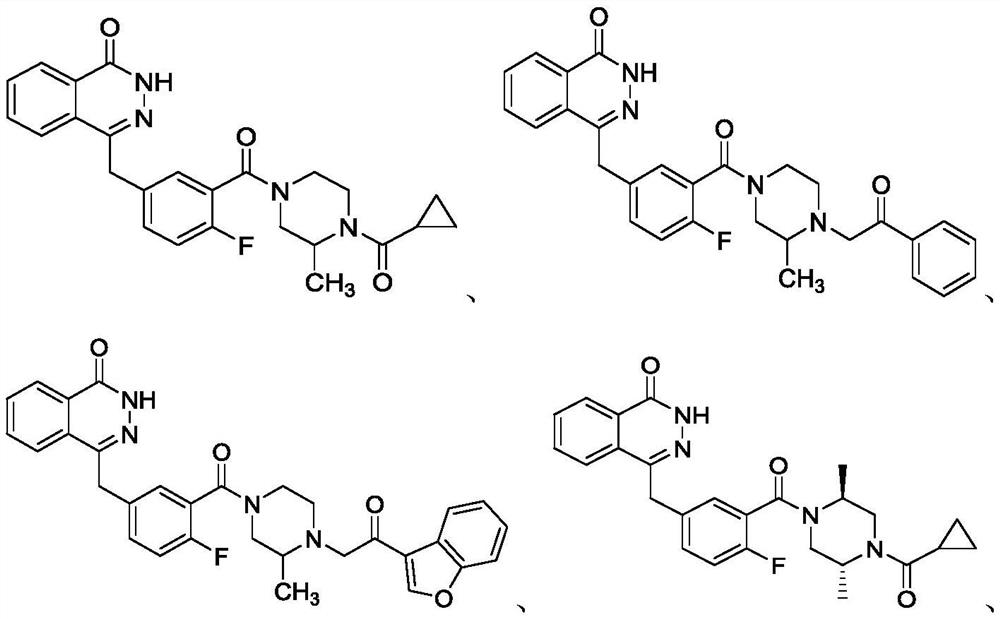
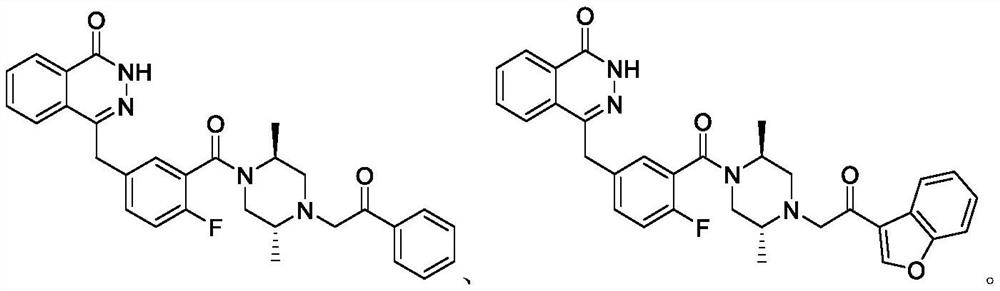
PARP inhibitor containing phthalazine-1 -(2H)-ketone structure as well as preparation method and medical application of PARP inhibitor
A pharmacy and drug technology, applied in the field of PARP inhibitors, can solve the problems of lack of specificity of the target, normal cell canceration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of 4-(3-(4-(cyclopropylcarbonyl)-3-methylpiperazine-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one (I-1)
[0034] 3-Methylpiperazine-1-carboxylic acid tert-butyl ester (Ⅲ-1)
[0035] (1.0g, 9.8mmol) 2-methylpiperazine (II-1) was dissolved in 20mL of dichloromethane (DCM), and (2.2g, 10.0mmol) Boc dissolved in DCM was added dropwise 2 O, the reaction was stirred at room temperature for 5h. TLC monitors that the reaction of the raw materials is complete, spin out the solvent under reduced pressure, add 40mL of water, extract with EA (3×20mL), combine the organic phases, wash the organic phases with saturated brine (3×50mL), dry over anhydrous sodium sulfate, filter, The filtrate was concentrated to give 1.7 g of a colorless oil. The crude product was purified by column chromatography (eluent, DCM:EA=1:1) to obtain 1.2 g of a colorless oil. Yield 60.0%. 1 H-NMR (300MHz, CDCl 3 )δ(ppm):3.95(brs,2H,-NC H 2 CH-,-C H CH 3 ), 2.96 (d, J=7.9Hz, 1H, -NC H 2 ...
Embodiment 2
[0042] 4-(4-fluoro-3-(3-methyl-4-(2-oxo-2-phenylethyl)piperazine-1-carbonyl)benzyl)phthalazin-1(2H)-one ( I-2) Synthesis
[0043] 3-Methyl-4-(2-oxo-2-phenylethyl)piperazine-1-carboxylic acid tert-butyl ester (Ⅳ-2)
[0044] Dissolve (250mg, 1.2mmol) tert-butyl 3-methylpiperazine-1-carboxylate (Ⅲ-1) in 10mL of acetonitrile, then add (248mg, 1.2mmol) 2-bromoacetophenone and (862mg, 6.2mmol) K 2 CO 3 , the temperature was raised to 80°C, and the reaction was stirred for 5h. TLC monitored the complete reaction of the raw materials, cooled to room temperature, filtered, washed the filter cake with EA, and concentrated the filtrate to obtain 400 mg of oil. The crude product was purified by column chromatography (eluent, PE:EA=9:1) to obtain 250 mg of a colorless oil. The yield was 63.0%. 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 8.03 (d, J = 7.4Hz, 2H, Ar H ), 7.61(t, J=7.3Hz, 1H, Ar H ), 7.49(t, J=7.5Hz, 2H, Ar H ), 4.18 (d, J=16.3Hz, 1H, -NC H 2 CHN-),3.97–3.60(m,3H,-COC H 2 ...
Embodiment 3
[0048] 4-(3-(4-(2-(benzofuran-3-yl)-2-oxoethyl)-3-methylpiperazine-1-carbonyl)-4-fluorobenzyl)phthalazine- 1(2H)-Kone(I-3)
[0049] Synthesis
[0050] tert-butyl 4-(2-(benzofuran-3-yl)-2-oxoethyl)-3-methylpiperazine-1-carboxylate (IV-3)
[0051] Dissolve (200mg, 1.0mmol) tert-butyl 3-methylpiperazine-1-carboxylate (Ⅲ-1) in 10mL DMF, and then add (239mg, 1.0mmol) 1-(benzofuran-3-yl )-2-Bromoethanone and (303mg, 3.0mmol) Et 3 N, the reaction was stirred at room temperature for 12 h, and the reaction of the raw materials was monitored by TLC to complete. Pour the reaction solution into 50 mL saturated NH 4 Cl solution, extracted with EA (3×15 mL), combined organic phases, washed with saturated brine (3×30 mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain 320 mg of yellow oil. The crude product was purified by column chromatography (eluent, PE:EA=9:1) to obtain 200 mg of light yellow oil. 1 H-NMR (300MHz, CDCl 3 )δ(ppm):8.81(s,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com