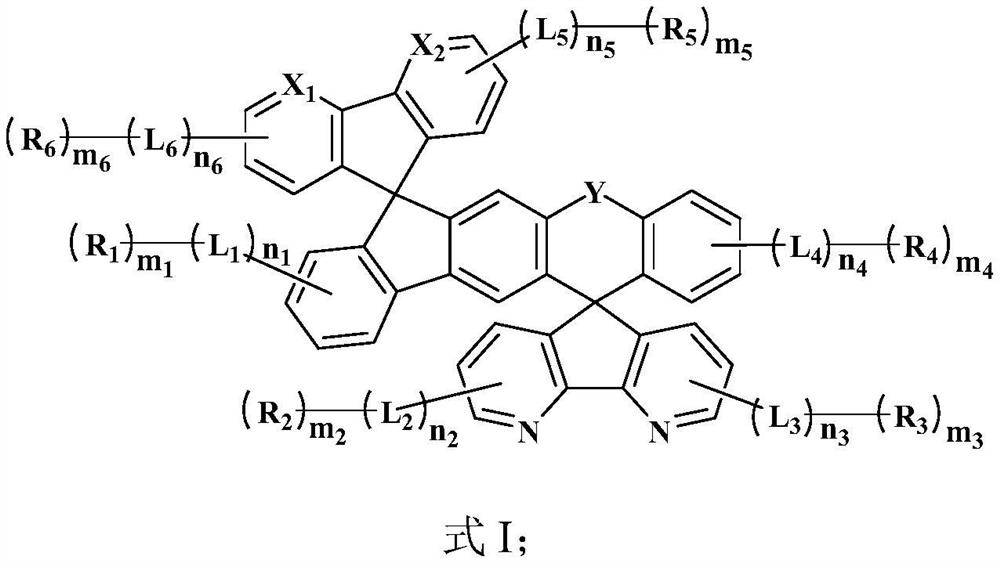
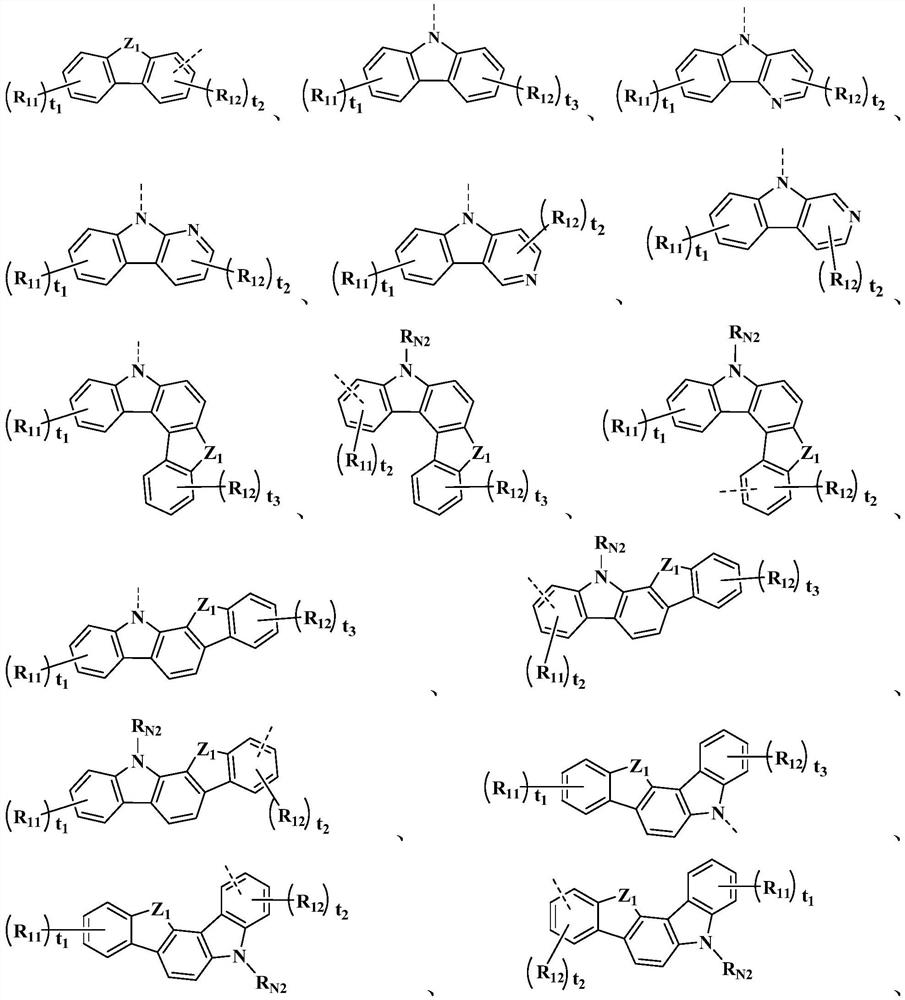
Organic compound and electroluminescent material and applications thereof
An organic compound, selected technology, applied in the field of organic compounds and electroluminescent materials, can solve the problems of phosphorescent host materials that cannot take into account energy consumption, efficiency, processability and life, low luminous efficiency and working life, and high turn-on voltage , to achieve the effect of reducing the injection barrier, improving the luminous performance and stability, and reducing the driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0114]
[0115] Under a nitrogen atmosphere, add about 100mL of anhydrous toluene to a 250mL reaction flask, then add reactant A1 (4mmol), reactant 1-1 (4mmol), sodium tert-butoxide t-BuONa (10mmol), palladium catalyst Pd 2 (dba) 3 (0.2 mmol) and the ligand 2-dicyclohexylphosphine-2',6'-dimethoxybiphenyl (S-Phos, 0.6 mmol), the temperature was raised to 110° C., and the reaction was carried out overnight. After the reaction is complete, cool to room temperature, add dichloromethane DCM / H 2 O was extracted, and the collected organic phase was washed with anhydrous Na 2 SO 4 After drying, the filtrate was collected by suction filtration, the solvent was spun off and purified by column chromatography to obtain intermediate B1 (yield 83%).
[0116] Characterization results of intermediate B1:
[0117] MALDI-TOF MS (m / z) obtained by matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis: C 31 h 19 BrO, calculated to be 486.06, found to be 486...
preparation example 2
[0122]
[0123] Under a nitrogen atmosphere, add the reaction solvent 1,2-dichlorobenzene in the reaction flask, add reactant a1 (6mmol), reactant 2-1 (7mmol), potassium carbonate (12mmol), catalyst CuI (0.6mmol) and Ligand 18-crown-6 (0.6mmol), heated to 180°C, reacted for 24h. After the reaction is completed, cool to room temperature, collect the organic phase by suction filtration, add DCM / H 2 O was extracted, and the collected organic phase was washed with anhydrous Na 2 SO 4 After drying, the filtrate was collected by suction filtration, the solvent was spun off and purified by column chromatography to obtain intermediate b1-1 (yield 71%).
[0124] Characterization results of intermediate b1-1:
[0125] MALDI-TOF MS (m / z) obtained by matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis: C 23 h 13 N 3 O, the calculated value is 347.11, and the measured value is 347.30.
[0126] The intermediates b1-2, b1-3, b1-4 and b1-5 were prep...
preparation example 3
[0131]
[0132] Under a nitrogen atmosphere, reactant 3-1 (3 mmol) was added to anhydrous tetrahydrofuran THF, stirred at -78°C to cool the reaction solution, and then 1.6M n-butyllithium n-BuLi (3 mmol) was added dropwise, And keep the reaction at -78°C for 2h; slowly drop the reactant A (3mmol) into the low-temperature reaction liquid, after the dropwise addition, continue the reaction at low temperature for 2h, then raise the temperature to room temperature and react overnight. After the reaction is completed, add a small amount of water to quench, add DCM / H 2 O was extracted, the organic phase was collected and washed with anhydrous Na 2 SO 4 Dry, collect the filtrate by suction filtration, and spin off the solvent to obtain the crude product;
[0133] The above crude product was added to 20 mL of acetic acid under nitrogen, stirred and heated, and reacted at 120° C. for 2 h, then added 2 mL of hydrochloric acid, and heated at this temperature for 12 h. After the rea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| internal quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com