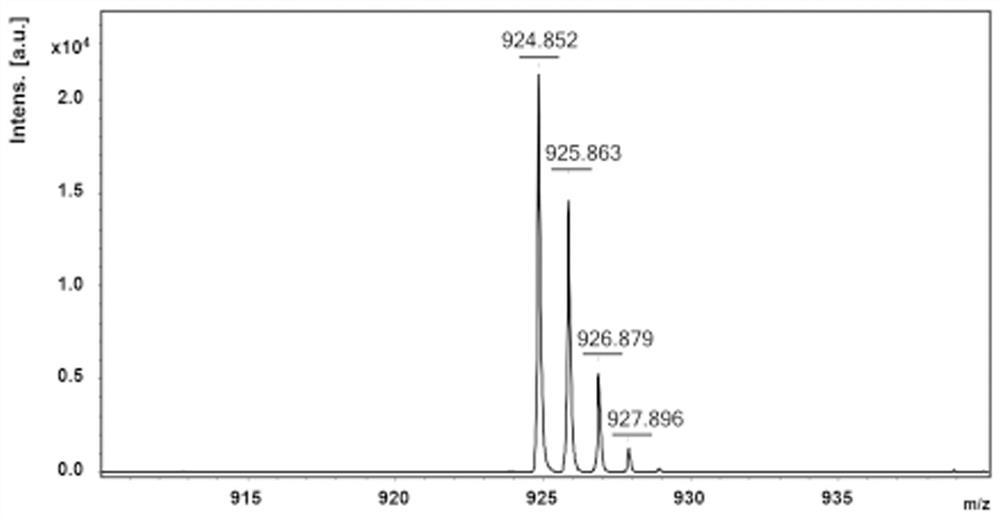
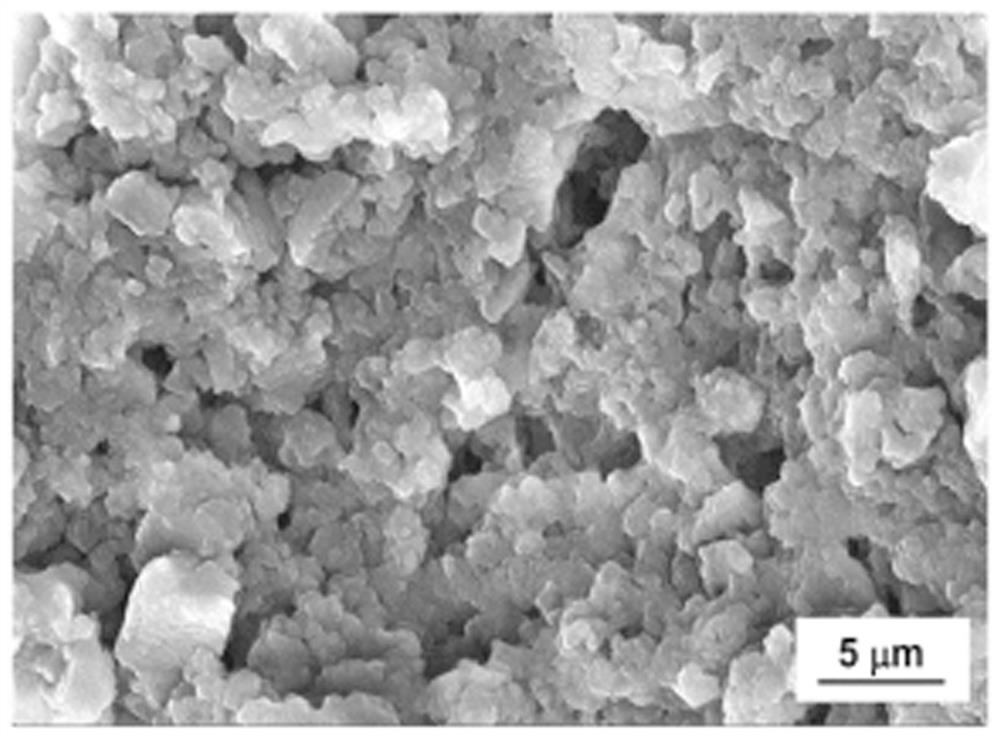
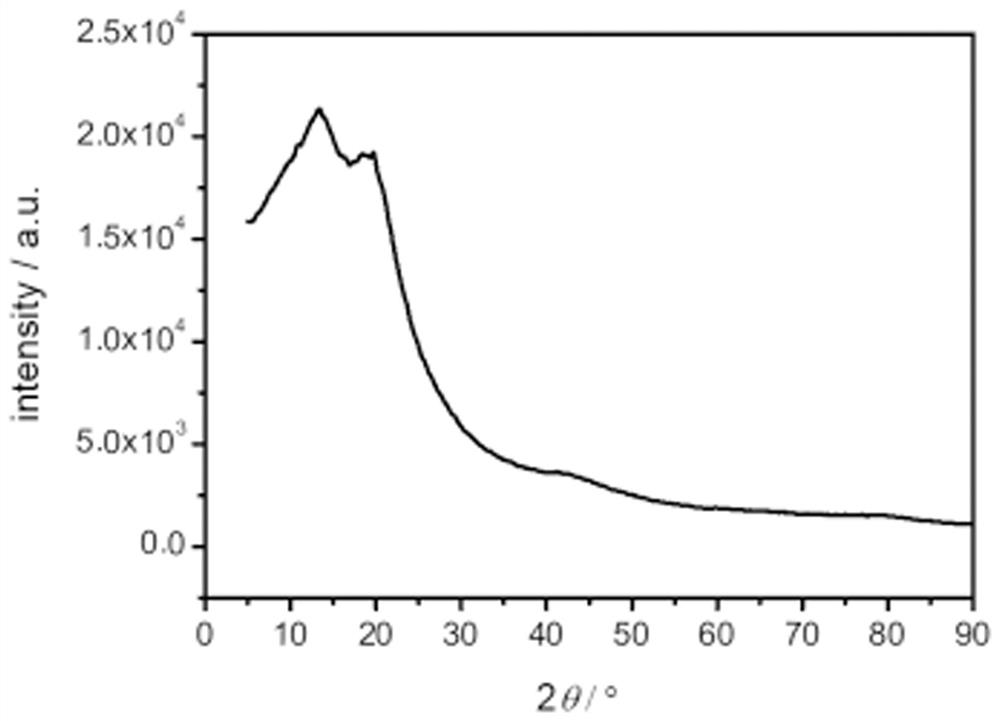
Polymer based on phenazine trimer, preparation method and battery application thereof
A phenazine trimer, battery application technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as dependence on scarce natural resources and low actual capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Synthesis of p-TPZB1
[0046] 1.TPZB (X=Z=H, Y=CH=CH 2 )Synthesis
[0047]
[0048] 5,10-dihydrophenazine (5.47g, 30mmol), 1,3,5-tribromobenzene (3.15g, 10mmol), 4-bromostyrene (5.49g, 30mmol), palladium acetate (202mg, 1.8mmol ), xPhos (1.72g, 3.6mmol), sodium tert-butoxide (8.65g, 90mmol) and toluene (30ml) were put into the reaction flask, and oxygen was removed by freezing-vacuum-thawing cycle 3 times. The mixture was condensed under reflux at 100° C. and stirred for 12 hours. After the obtained crude product was cooled to room temperature, it was purified by column chromatography with neutral alumina (DCM:PE=1:2 to 1:1), and then recrystallized (mixture of DCM and MeOH). 1 H NMR (400MHz, C6D6, ppm) δ = 7.28 (d, J = 8.4Hz, 6H), 7.18 (d, J = 3.3Hz, 9H), 6.56 (dd, J = 17.6, 10.9Hz, 3H), 6.38 (dtd, J=23.1,7.5,1.4Hz,12H),6.15(dd,J=7.7,1.4Hz,6H),5.93(dd,J=7.8,1.4Hz,6H),5.61(d,J=17.5 Hz,3H),5.14(d,J=11.1Hz,3H).13C NMR(101MHz,C6D6,ppm)δ=146.37,139.71,137...
Embodiment 2
[0051] Embodiment 2: the synthesis of TPZB-6COOLi
[0052]
[0053] 1. Synthesis of TPZB-6COOMe
[0054] 5,10-dihydrophenazine (2.73g, 15mmol), 1,3,5-tribromobenzene (1.57g, 5mmol), dimethyl 5-bromo-isophthalate (4.1g, 15mmol), acetic acid Palladium (100mg, 0.9mmol), xPhos (860mg, 1.8mmol), cesium carbonate (14.7g, 90mmol) and . Toluene (30ml) was put into the reaction bottle, and the oxygen was removed by freezing-vacuum-thawing cycle 3 times. The mixture was refluxed and condensed at 130°C for 12 hours with stirring. The obtained crude product was quenched with methanol, cooled to room temperature, purified by column chromatography with neutral alumina (PE:EA=3:1), and recrystallized (mixture of DCM and MeOH). 1 H NMR (400MHz, C6D6, ppm) δ = 9.05 (t, J = 1.6Hz, 1H), 8.42 (d, J = 1.6Hz, 2H), 7.32 (s, 1H), 6.43 (td, J = 7.8, 1.3Hz, 3H), 6.31(td, J=7.8, 1.3Hz, 3H), 6.21(dd, J=7.9, 1.2Hz, 3H), 5.81(dd, J=7.9, 1.2Hz, 3H), 3.41( s,6H).
[0055] 2. Synthesis of TPZB-6COOLi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com