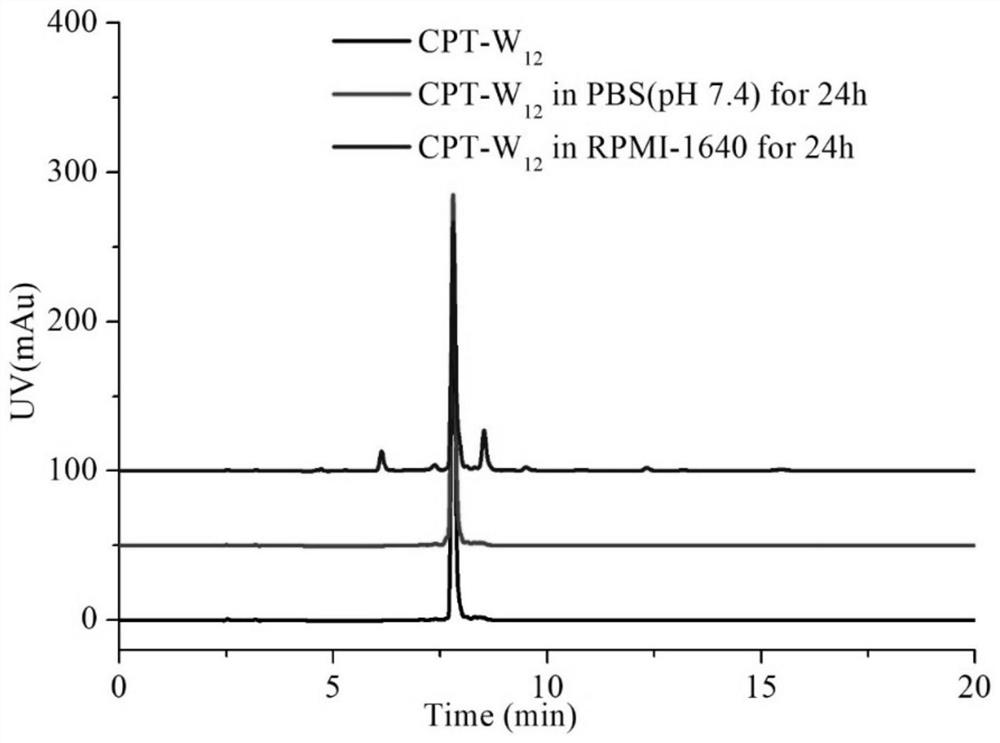
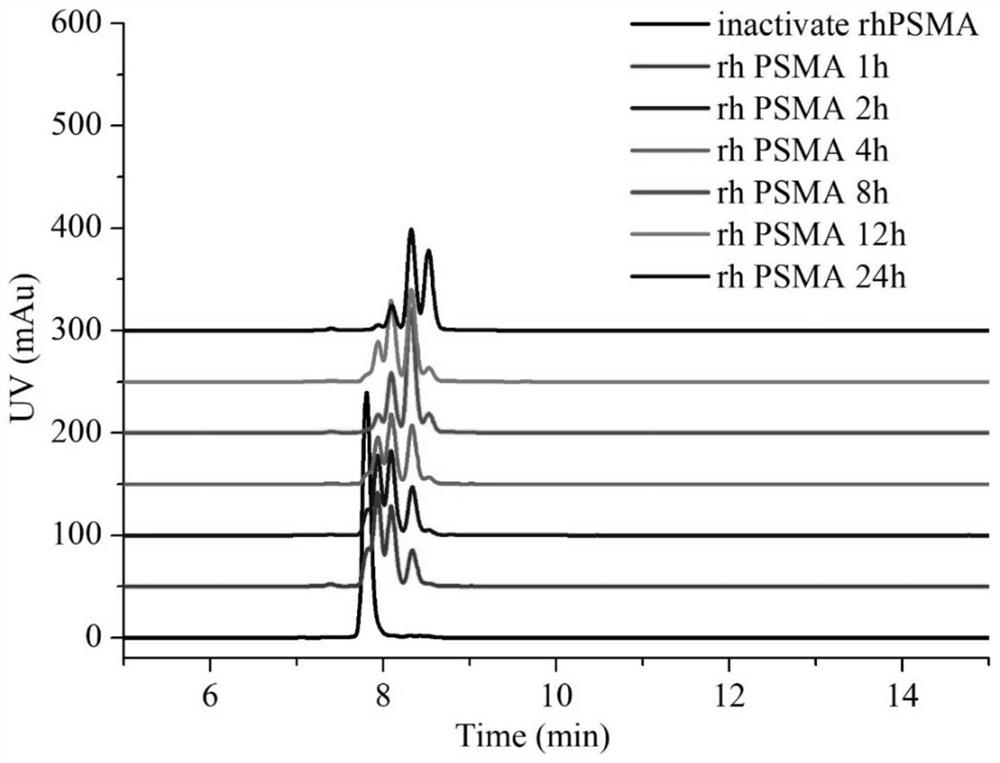
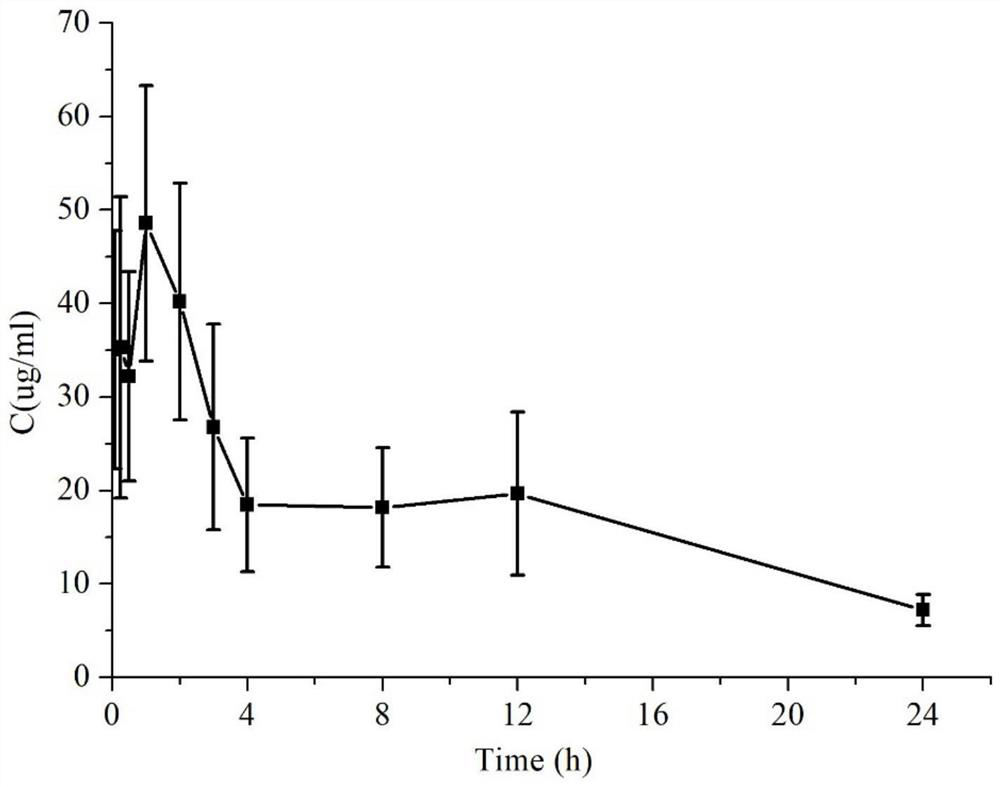
PSMA activated anti-tumor prodrug CPT-Y and preparation method and application thereof
An anti-tumor effect, CPT-W12 technology, used in anti-tumor drugs, pharmaceutical formulations, drug combinations, etc., can solve the problems of toxic side effects, poor targeting, and poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0213] The preparation of embodiment 1 intermediate WT-A
[0214] Sequentially weigh 500mg (1.18mmol) 1-tert-butyl fluorenylmethoxycarbonyl-L-glutamate, 338mg (1.76mmol) EDCI and 238mg (1.76mmol) HOBt into a 50mL single-necked bottle, add 20mL of anhydrous dichloro Make it dissolve with methane, stir and react at room temperature for 0.5h, continue to add 382mg (1.29mmol) L-di-tert-butyl glutamate hydrochloride and 380mg (2.94mmol) DIPEA, stir at room temperature for 4h, TLC [V (petroleum ether ): V (ethyl acetate) = 2: 1] to monitor the complete reaction, the reaction solution was diluted to 50mL with dichloromethane, washed three times with 25mL of 1M hydrochloric acid solution, then washed with 25mL of saturated sodium bicarbonate solution, and then Dehydrate with saturated brine, collect the organic layer, dry the organic layer with an appropriate amount of anhydrous sodium sulfate, filter, evaporate to dryness under reduced pressure, mix the sample with silica gel, flash ...
Embodiment 2
[0215] The preparation of embodiment 2 intermediate WT-B
[0216] Weigh 600mg (0.90mmol) of WT-A into a 50mL single-necked bottle, add 20mL of anhydrous DMF and ultrasonically dissolve it, then add 229mg (2.70mmol) of piperidine, stir and react at room temperature for 0.5h, TLC [V (petroleum ether ): V (ethyl acetate) = 2: 1] to monitor the complete reaction, the reaction solution was directly spin-dried by a diaphragm pump, then added 50 mL of ethyl acetate to dissolve, and the organic layer was washed 3 times with 30 mL of distilled water each time, and dehydrated with saturated saline. Dry over sodium sulfate, filter, mix with silica gel, flash silica gel column chromatography (PE / EA=3 / 1 to 0 / 1), and obtain 340 mg of colorless oil WT-B. Yield: 85.0%; 1 H NMR (500MHz, CDCl 3 ):δ1.40(d,27H,9×-CH 3 ),1.79(m,2H),2.03(m,2H),2.19(m,2H),2.32(m,2H),3.29(m,1H),4.42(m,1H),6.62(d,J= 7.3Hz,1H); 13 C NMR (125MHz, CDCl 3 ):δ27.8,28.0,28.1,30.3,31.6,32.9,52.3,54.5,80.7,81.3,82.2,171...
Embodiment 3
[0217] The preparation of embodiment 3 intermediate WT-C
[0218] Sequentially weigh 200mg (0.47mmol) 1-tert-butyl fluorenylmethoxycarbonyl-L-glutamate, 135mg (0.71mmol) EDCI and 95mg (0.71mmol) HOBt into a 50mL single-necked bottle, add 10mL of anhydrous dichloro Make it dissolve with methane, stir and react at room temperature for 0.5h, continue to add 230mg (0.52mmol) WT-B and 152mg (1.18mmol) DIPEA, stir at room temperature for 4h, TLC [V (petroleum ether): V (ethyl acetate) = 1:1] to monitor the completion of the reaction, dilute the reaction solution to 50mL with dichloromethane, wash with 25mL of 1M hydrochloric acid solution three times each time, then wash with 25mL of saturated sodium bicarbonate solution, then dehydrate with saturated saline, and collect the organic layer , the organic layer was dried with an appropriate amount of anhydrous sodium sulfate, filtered, evaporated to dryness under reduced pressure, mixed with silica gel, flash silica gel column chromato...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com