Composite solid electrolyte, preparation method thereof and application thereof in solid-state secondary battery
A solid electrolyte and electrolyte technology, which is applied in the manufacture of solid electrolytes, non-aqueous electrolyte batteries, and electrolyte batteries, etc., can solve the problems of difficult processing, hindering the conduction of working ions, and limited enhancement of lithium ion conduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
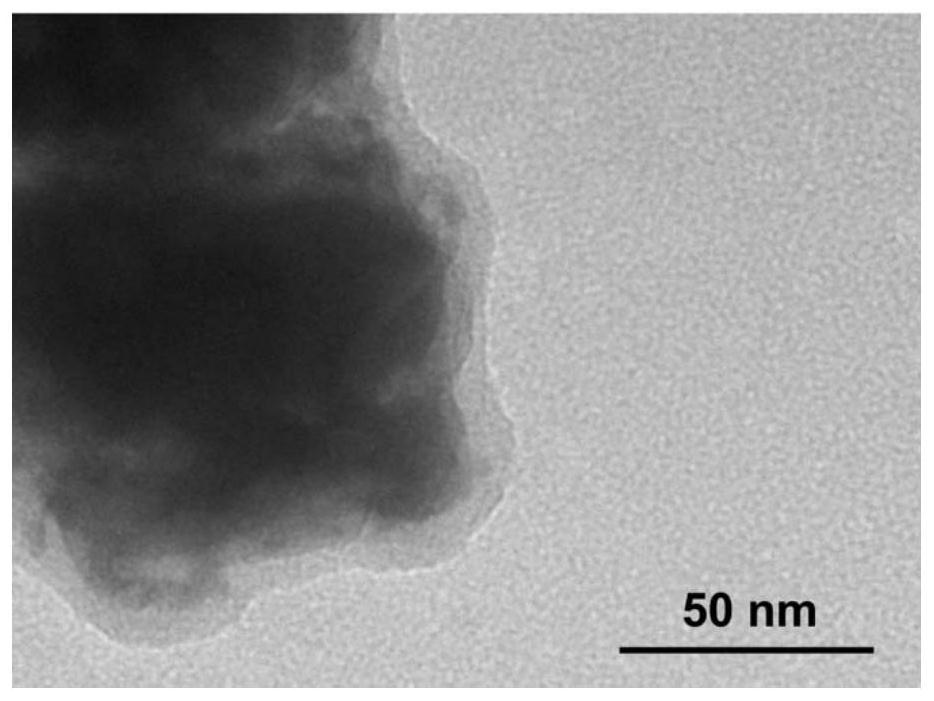
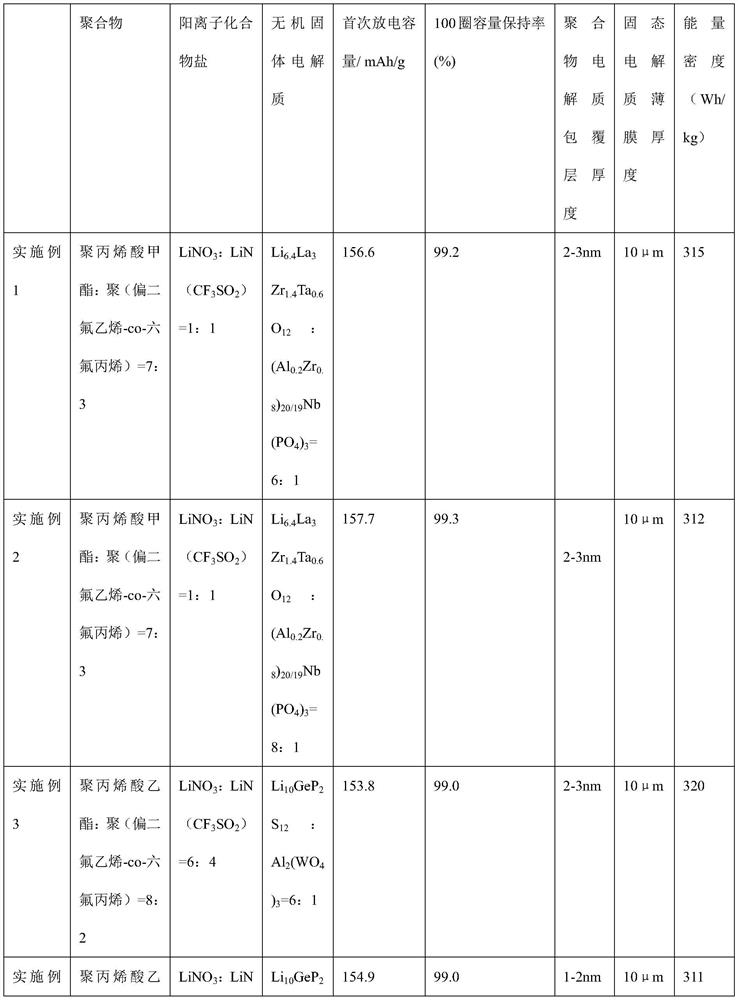
[0056] (1) Preparation of conductive coated composite solid electrolyte: polymethyl acrylate and poly(vinylidene fluoride-co-hexafluoropropylene) were mixed according to the mass ratio of 7:3, and then lithium nitrate and LiN(CF 3 SO 2 ) is mixed according to the mass ratio of 1:1, the polymer and the cationic compound salt are mixed uniformly at the molecular level according to the mass ratio of 5:1, heated and stirred to form a precursor solution A; the inorganic solid electrolyte Li 6.4 La 3 Zr 1.4 Ta 0.6 o 12 :(Al 0.2 Zr 0.8 ) 20 / 19 Nb(PO 4 ) 3 Mix according to the Li:Al molar ratio of 6:1, add the inorganic electrolyte mixture powder into the precursor solution A, control the amount of the inorganic electrolyte mixture powder added to the polymer mass ratio of 98:2, and mix thoroughly and uniformly to form the precursor solution B; add The method of precipitating the polymer with a volume fraction of 10% isopropanol was used to prepare a composite solid electroly...
Embodiment 11
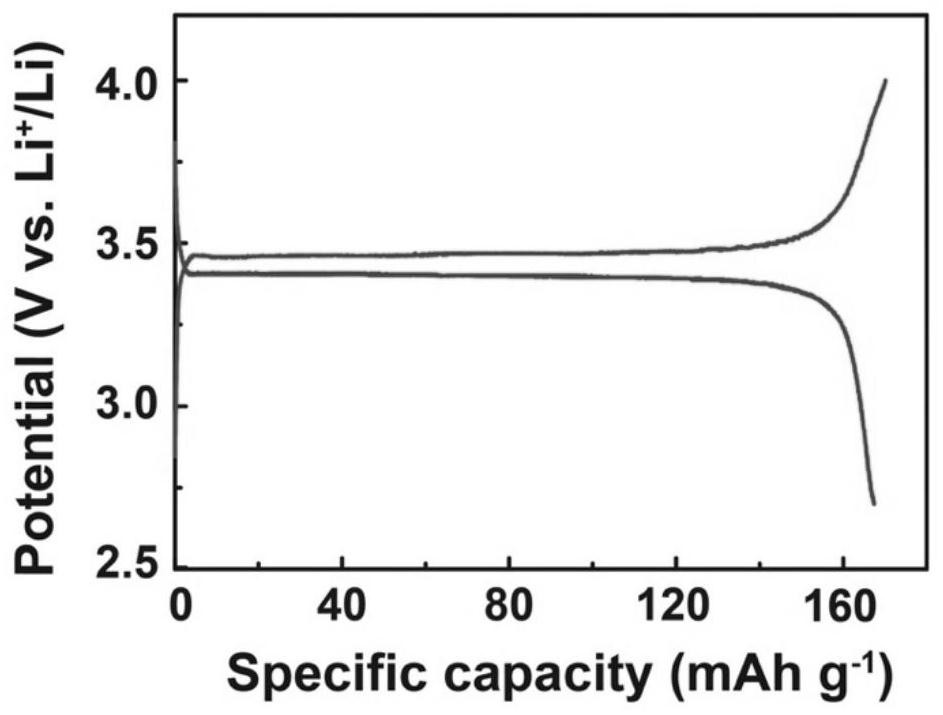
[0062] Other process parameters are different from Example 1, the only difference is: the mass ratio of inorganic solid electrolyte powder to polymer is 90:10, and the preparation process of Example 1 is used to obtain a conductive coated composite solid electrolyte, and the experimental data of charge and discharge cycles at room temperature See Table 1.
Embodiment 12
[0064] (1) Preparation of conductive coated composite solid electrolyte: polymethyl acrylate and polyvinylidene fluoride are mixed according to the mass ratio of 8:2 to obtain a polymer mixture, lithium difluorooxalate borate and lithium nitrate = 1:1 according to The mass ratio is 7:1 to obtain a mixed cationic compound salt, and the polymer and the cationic compound salt are mixed uniformly at the molecular level, heated and stirred to form a precursor solution A; according to the ratio of molar ratio Li:Al=8:1, Li 10 GeP 2 S 12 and Al 2 (WO 4 ) 3 The inorganic solid electrolyte mixed powder is added to the precursor solution A, and the mass ratio of the inorganic electrolyte mixture powder to the polymer is controlled to be 95:5, and the precursor solution B is fully and uniformly mixed; the volume fraction of 8% ethanol is added to precipitate the polymer. Methods A conductive coated composite solid electrolyte was prepared. Dry in an oven at 80 degrees for 24 hours. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com