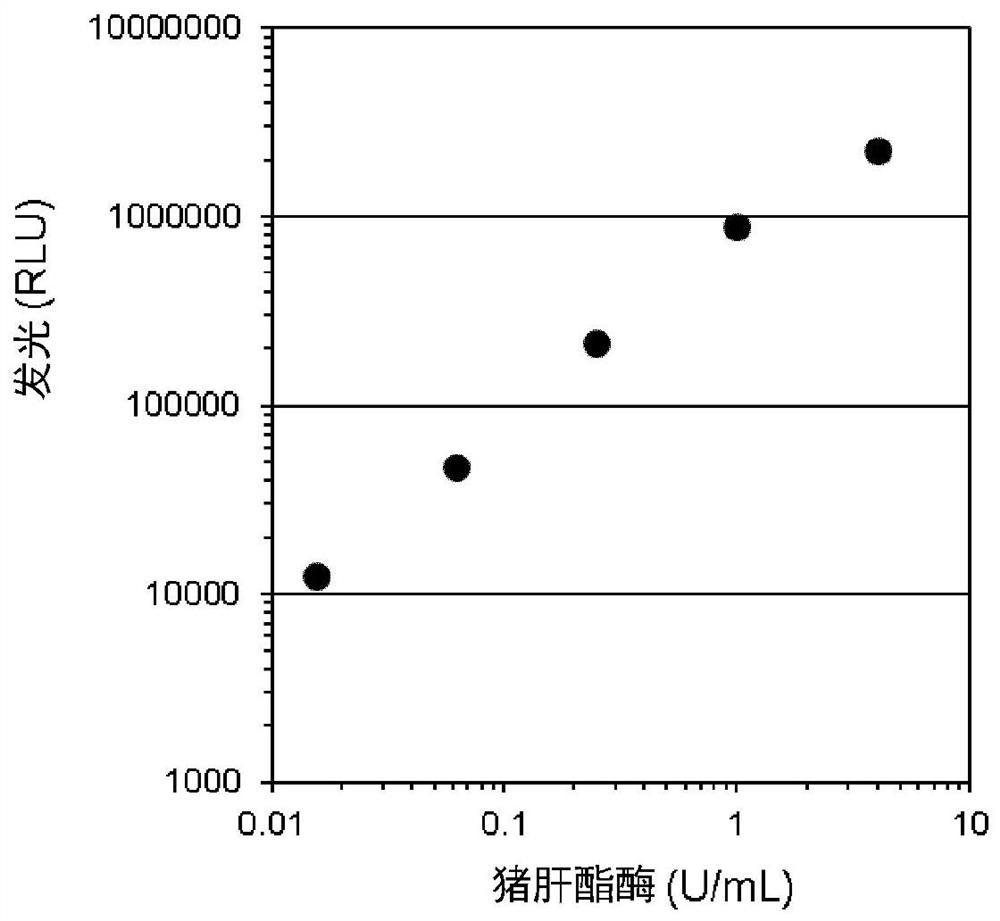
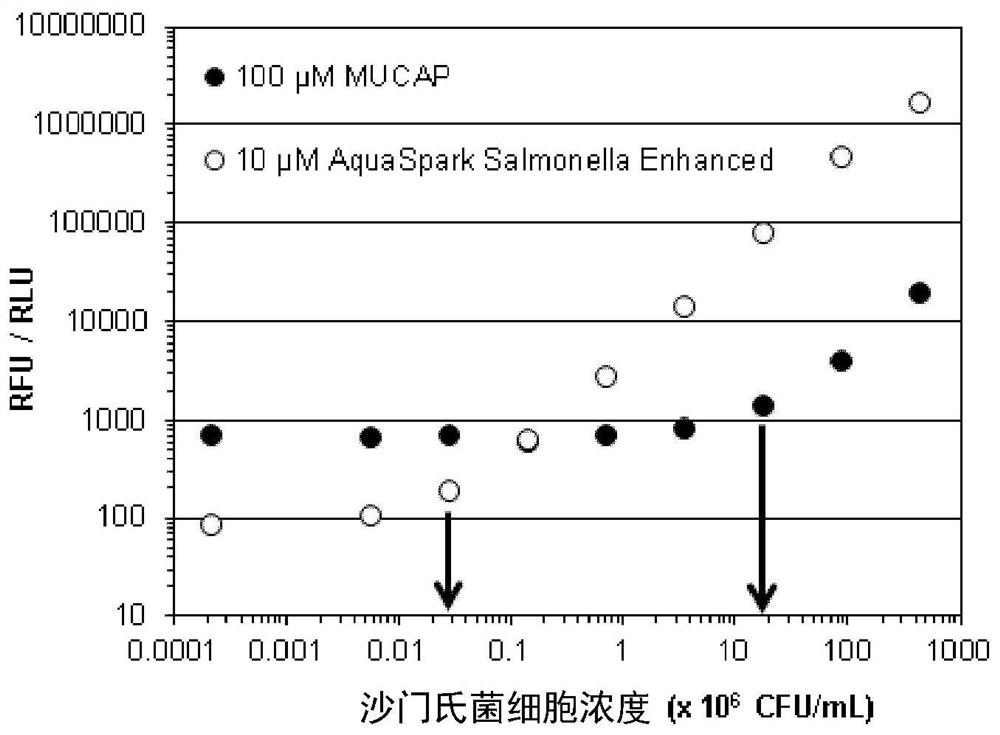
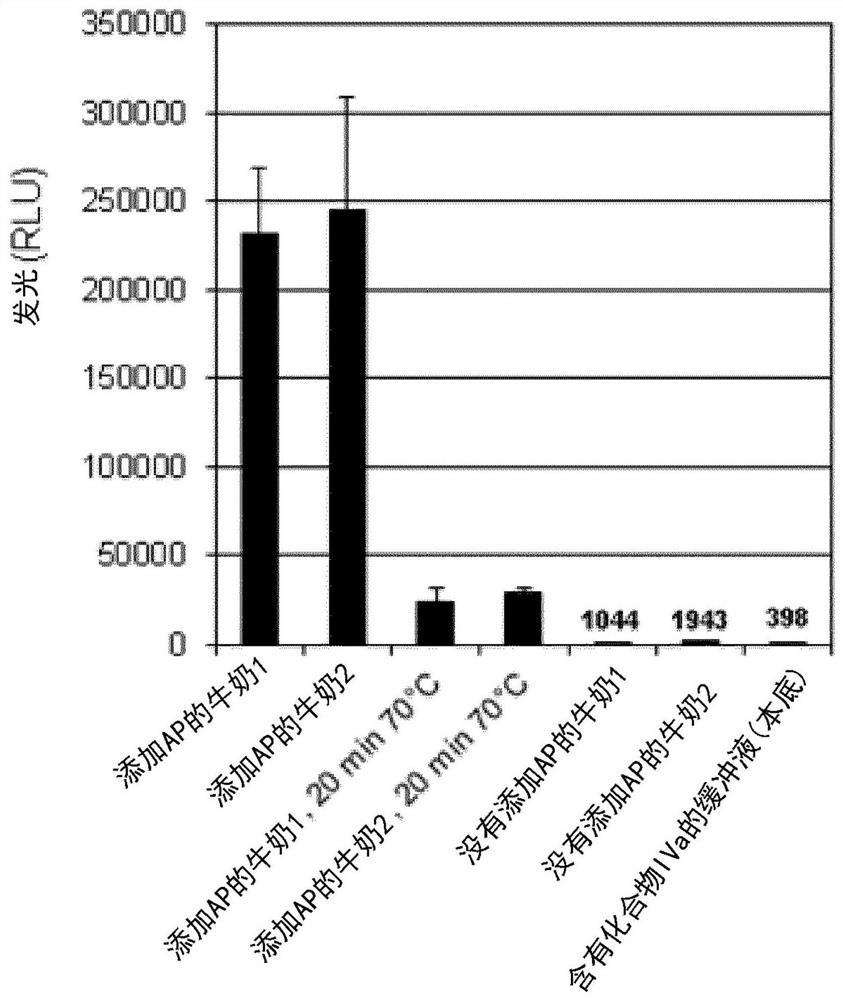
Dioxetane compounds and their use for the detection of microorganisms
A technology of compounds and heterocycles, applied in the field of dioxetane compounds and their use in the detection of microorganisms, can solve the problems of limited shelf life, complexity, and high cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0352] General method:
[0353] All reactions were performed at room temperature unless otherwise stated. Chemicals and solvents were A.R. grade or purified by standard techniques. Thin Layer Chromatography (TLC): Silica gel plates Merck 60F254: Compounds were visualized by irradiation with UV light. Column chromatography (FC): silica gel Merck 60 (particle size 0.040-0.063 mm), eluents are given in parentheses. Reverse phase high pressure liquid chromatography (RP-HPLC): C18 5 μm, 250×4.6 mm, eluents are given in brackets. Preparative RP-HPLC: C18 5 μm, 250×21 mm, eluents are given in brackets. Fluorescence and chemiluminescence were recorded on a Molecular Devices Spectramax i3x.
[0354] All chemicals were purchased from Merck and Biosynth AG and used as received if not stated otherwise.
[0355] Abbreviation. AcOH-acetic acid, MeCN-acetonitrile, DCM-dichloromethane, DMF-N,N'-dimethylformamide, EtOAc-ethyl acetate, Hex-hexane, MeOH-methanol, TFA-trifluoroacetic acid, ...
Synthetic example 1
[0356] Synthesis Example 1: Synthesis of Compound IIa
[0357]
[0358] DCC (457 mg, 2.21 mmol, 1.1 eq) was added to a mixture of octanoic acid (350 μl, 2.21 mmol, 1.1 eq) and 4-hydroxybenzyl alcohol (250 mg, 2.01 mmol, 1 eq) in DCM (2 ml). The reaction mixture was stirred at room temperature and monitored by TLC (40:60 EtOAc:Hex). Upon completion, DCC was filtered off and the crude product was purified by column chromatography on silica gel (30:70 EtOAc:Hex) to give compound 1a (246 mg, 49% yield) as a pale yellow solid. 1 H NMR (400MHz, CDCl 3 )δ7.35(d, J=8.5Hz, 2H), 7.05(d, J=8.5Hz, 2H), 4.63(s, 2H), 2.55(t, J=7.5Hz, 2H), 1.76(dt, J=15.1, 7.5Hz, 2H), 1.50-1.18(m, 8H), 0.97-0.82(m, 3H). 13 C NMR (101MHz, CDCl 3 )δ 173.75, 150.68, 133.71, 129.49, 121.78, 65.50, 34.48, 31.72, 29.16, 29.14, 29.11, 28.98, 25.01, 22.67, 14.13.
[0359]
[0360] Compound la (200 mg, 0.8 mmol, 1 eq) was dissolved in 4 mL of ACN and cooled to 0 °C. Sodium iodide (360 mg, 2.4 mmol, 3 eq) ...
Synthetic example 2
[0365] Synthesis Example 2: Synthesis of Compound IIIa
[0366]
[0367] 1,2:4,5-Di-O-isopropylidene-inositol (250 mg, 0.96 mmol, 1 eq) and imidazole (98 mg, 1.44 mmol, 1.5 eq) were dissolved in dry pyridine (3 ml) and cooled to -10°C. Tert-butyldiphenylchlorosilane (275 μl, 1.06 mmol, 1.1 eq) was added slowly via syringe. The reaction was allowed to warm to room temperature and monitored by TLC (50:50 EtOAc:Hex). Upon completion, the reaction mixture was diluted with EtOAc and washed with saturated NH 4 Cl wash. The organic layer was separated, washed with Na 2 SO 4 Dry, filter and evaporate the solvent under reduced pressure. The crude product was purified by column chromatography on silica gel (50:50 EtOAc:Hex) to afford compound la (350 mg, 73% yield) as a white foam. MS (ES+): C 28 h 38 o 6 m / z calculated for Si: 498.24; found: 499.4 [M+H] + .
[0368]
[0369] Compound 1a (170 mg, 0.341 mmol, 1 eq) was dissolved in DCM and ethyl vinyl ether (652 μl, 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com