Use of riluzole oral disintigrating tablets for treating diseases
A technology for riluzole and diseases, applied in muscular system diseases, nervous system diseases, neuromuscular system diseases, etc., can solve problems such as complex ability, low solubility, and dysphagia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] A phase 1 study to evaluate the bioequivalence between BHV-0223 (riluzole 40mg sublingual orally disintegrating tablet) and RILUTEK 50mg tablet and to evaluate the food effect of BHV-0223 in normal healthy volunteers
[0100] This study is sometimes referred to herein as BHV223-102. The main elements of the protocol used in the study are as follows.
[0101] Target
[0102] main target
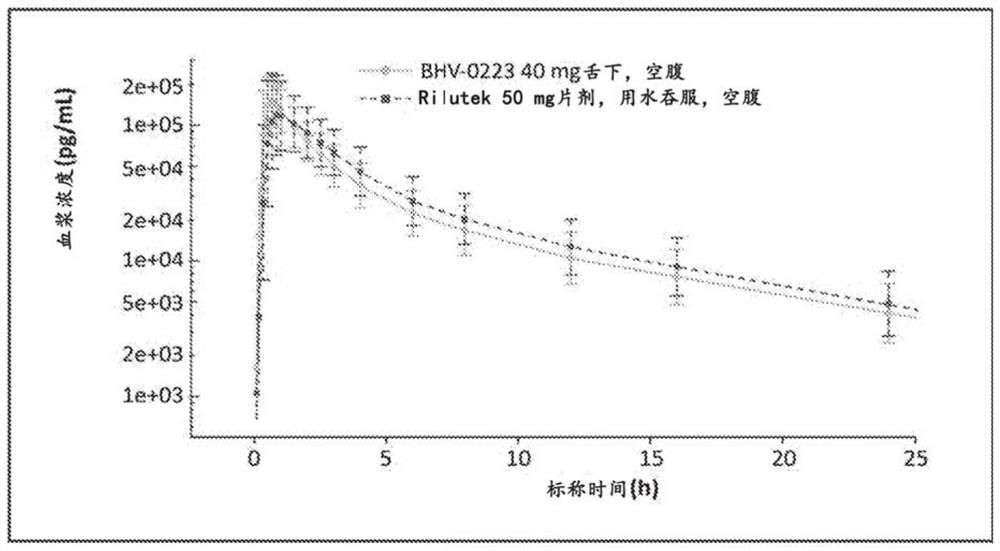
[0103] Comparing the rate and extent of absorption of BHV-0223 administered sublingually at 1 x 40 mg ODT in NHV with RILUTEK administered orally as 1 x 50 mg tablet under fasted conditions.
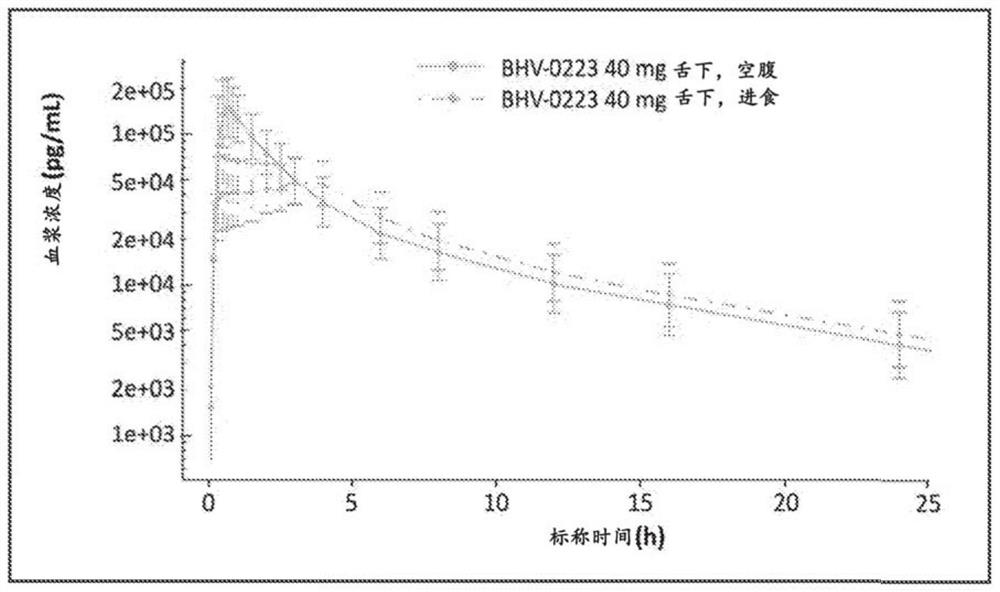
[0104] • To evaluate the effect of food on the pharmacokinetics of BHV-0223 when given as a single 40 mg sublingual dose in NHV.
[0105] secondary goal
[0106] · To evaluate the safety and tolerability of BHV-0223.
[0107] • To assess the rate of sublingual absorption of crushed riluzole tablets (50 mg RILUTEK) in a subset of NHVs.
[0108] explore target
[0109] To explore the sy...
Embodiment 2
[0212] The research result of embodiment 1
[0213] The study (BHV223-102) was performed substantially as described in the protocol set forth in Example 1. The results are summarized below.
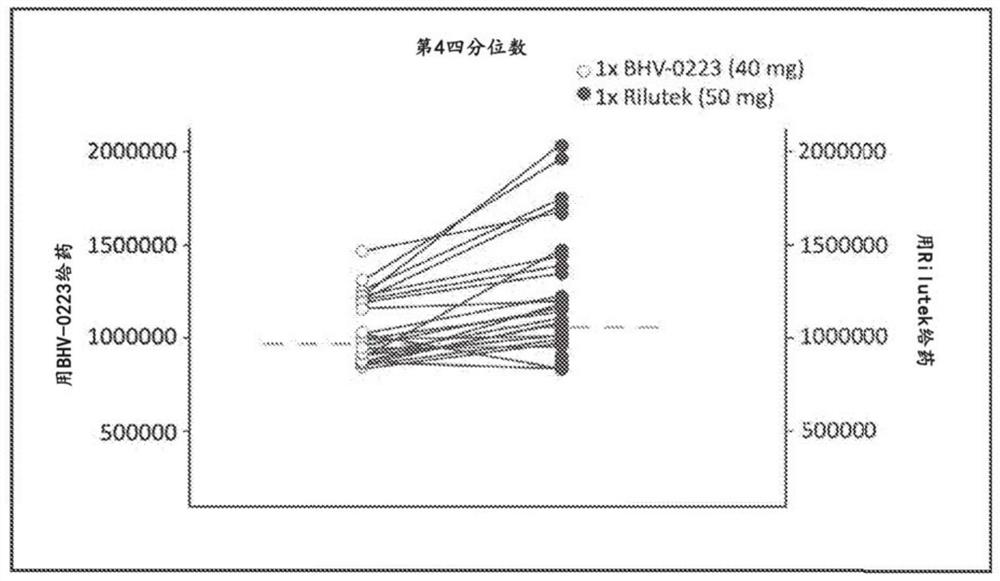
[0214] Results: In Part I, BHV-0223 achieved approximately 90% and 113% area under the curve (AUC) and maximum concentration exposure compared to RILUTEK, respectively. The 90% confidence interval is within the range of 80%-125% that FDA requires for bioequivalence. BHV-0223 produced AUC levels with a fed to fasted ratio of 92%. Crushed sublingual RILUTEK delivery had a 6% rate AUC level compared to oral RILUTEK.
[0215]SUMMARY / CONCLUSION: BHV-0223 is bioequivalent to RILUTEK 50mg oral tablet and therefore offers similar efficacy; but also potentially improves usability and reduces patient burden (no swallowing required, no need for fasting based on AUC) negative food effects); improved safety / tolerability (reduced risk of dose-related hepatic dysfunction); and enhanced pharmacology ...
Embodiment 3
[0334] Simulation and Modeling
[0335] Part A
[0336] use Modeling software to evaluate BHV-0223 40MG Effect of Sublingual Formulation and Riluzole 50MG Oral Tablet on Liver Function Test Parameters
[0337] The main elements of the simulation are summarized below.
[0338] Target
[0339] To quantitatively and mechanistically compare the hepatotoxicity potential of oral riluzole to BHV-0223 using DILIsym, combining clinical and mechanistic data. DILIsym is a registered trademark of Dilisym Services Inc., Durham, NC, USA.
[0340] method
[0341] Oral administration of riluzole (50 mg twice daily [BID] for 12 weeks) and sublingual riluzole (40mg BID for 12 weeks).
[0342] • The DILIsym PBPK model framework for Riluzole is composed of a compartment model of the human body with compartments for blood, intestine, liver, muscle and other tissues.
[0343] The PBPK representation of riluzole is based on available data from BHV-0223 and published riluzole studies...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com