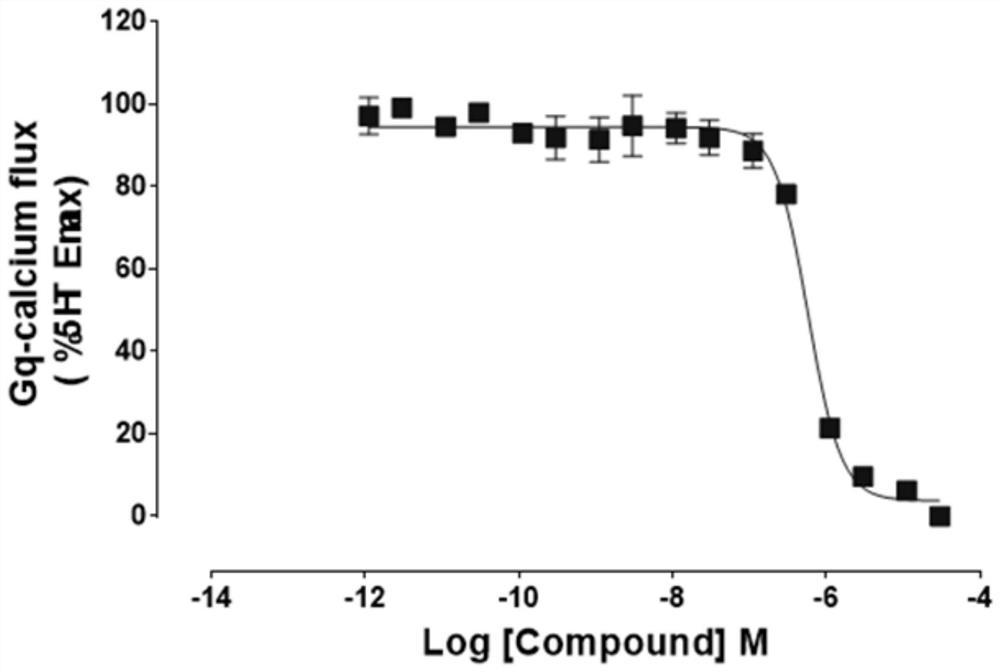
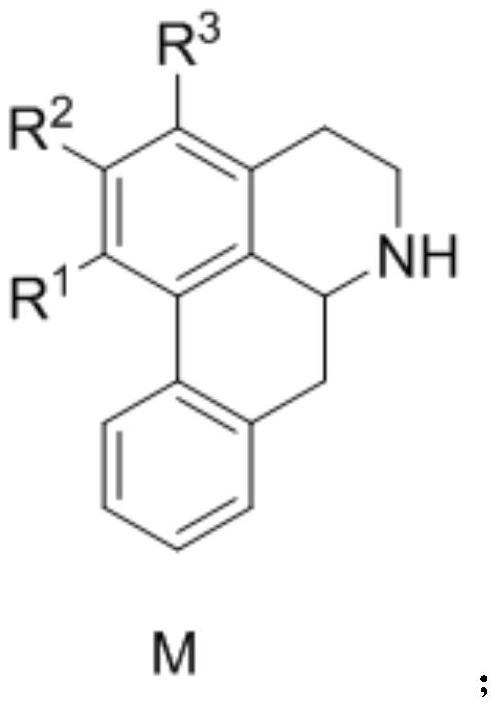
Application of aporphine compound
A compound and hydrate technology, applied in the field of pharmacy, can solve problems such as unidentified agonists, unknown functions, unknown receptor agonists, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the synthesis of compound S1
[0054] The synthetic route of compound S1 is as follows:
[0055]
[0056] Among them, the reaction conditions: (a) HOBt, EDC, DCM, rt (room temperature); (b) P 2 o 5 , toluene, 110°C; NaBH 4 , MeOH, 0°C-rt (c) TEA, TFAA, DCM, 0°C-rt; (d) PhDavePhos (2-diphenylphosphine-2'-(N,N-dimethylamino)biphenyl), Pd (OAc) 2 , K 2 CO 3 , DMA, 130°C; (e) NaBH 4 , EtOH, N 2 , rt.
[0057]Synthesis of compound S1-C: 2.93mL (20mmol) of commercially available 4-methoxyphenethylamine (S1-A) and 4.73g (22mmol) of o-bromophenylacetic acid (S1-B) were dissolved in 100mL of anhydrous di In methyl chloride, 2.97 g HOBt (22 mmol), 4.22 g EDC (22 mmol) were added with stirring, and the resulting reaction mixture was stirred at room temperature overnight. The mixture was separated by extraction with dichloromethane and water, and the organic phase was washed successively with 1N dilute hydrochloric acid, saturated sodium bicarbonate and s...
Embodiment 2
[0062] Embodiment 2: the synthesis of compound S1 (R)
[0063] The compound S1-C prepared in Example 1 and the dehydrating agent were placed in toluene and refluxed for 1.5 hours. A white precipitate was precipitated in the solution. After it became a yellow solid, TLC detected that there was no raw material in the toluene, and the toluene was poured out. The solid was saturated with Sodium bicarbonate and ethyl acetate work up. Separated by extraction, the organic phase was concentrated under reduced pressure. After the ring-closing mixture was purified by column chromatography, 280 mg (848 mmol) of the above intermediate was dissolved in 2 mL of DMF, and 0.028 mmol of RuCl[(s,s)-TsDPEN](p-cymene) (S / C=30 ), add the azeotrope of formic acid and triethylamine in total 1mL (molar ratio = 5:2) under ice bath, and gradually raise to room temperature under ice bath and stir for 12h after the addition. Basified with sodium bicarbonate, extracted with dichloromethane, washed with ...
Embodiment 3
[0065] Embodiment 3: the synthesis of compound S1 (S)
[0066] The compound S1-C prepared in Example 1 and the dehydrating agent were placed in toluene and refluxed for 1.5 hours. A white precipitate was precipitated in the solution. After it became a yellow solid, TLC detected that there was no raw material in the toluene, and the toluene was poured out. The solid was saturated with Sodium bicarbonate and ethyl acetate work up. Separated by extraction, the organic phase was concentrated under reduced pressure. After the mixture obtained by ring closure was purified by column chromatography, 280 mg (848 mmol) of the above intermediate was dissolved in 2 mL of DMF, and 0.028 mmol of RuCl[(R,R)-TsDPEN](p-cymene) was added (S / C=30) 1 mL of the azeotrope of formic acid and triethylamine (molar ratio = 5:2) was added under ice-cooling, and after the addition was completed, it was gradually raised to room temperature under ice-cooling and stirred for 12 h. Basified with sodium bic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com