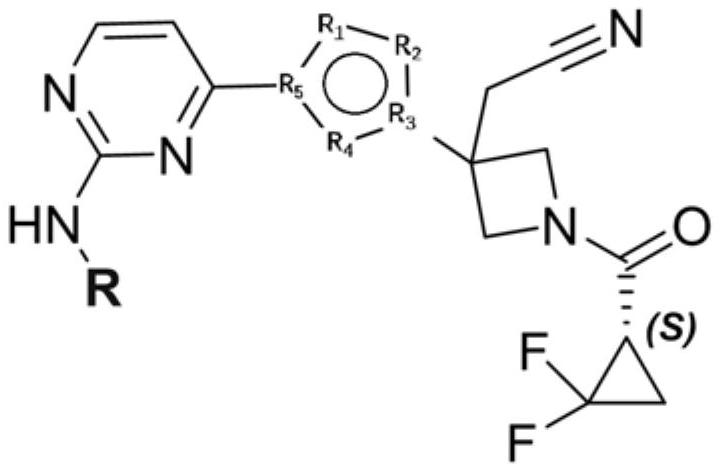
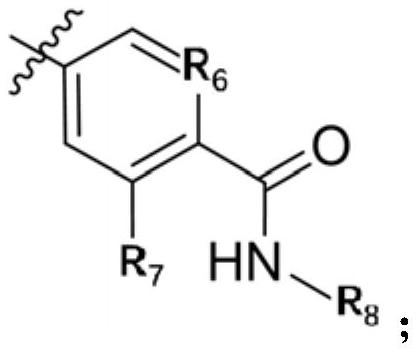
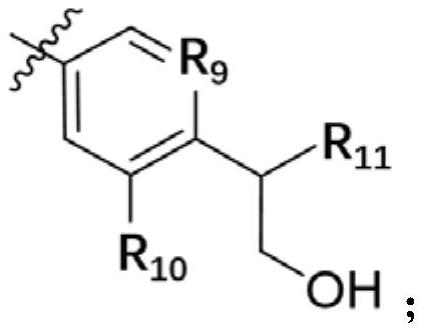
A kind of small molecular compound as jak kinase inhibitor and its use
A small molecule compound, compound technology, applied in the direction of anti-inflammatory agent, drug combination, organic chemistry, etc., to achieve good JAK kinase activity and effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 The General Method for Synthesizing Compound 1 (TDM-180973)
[0076]
[0077] Step 1: Preparation of compound 1c (2-chloro-4-(1H-pyrrol-3-yl)pyrimidine)
[0078] Add compound 1a (2g, 13.43mmol) to 250mL three-necked flask, compound 1b (4.69g, 13.43mmol), tetrakis (triphenylphosphine) palladium (940mg, 1.08mmol), potassium carbonate (3.7g, 26.85mmol), Dioxane (120 mL) and water (120 mL). The reaction liquid was replaced with nitrogen several times, heated to 80°C for 45 minutes, LCMS [M+H] +=180, the detection reaction is complete. Post-processing: the reaction solution was concentrated and dried, and the obtained crude product was passed through the column [eluent: (EA / PE)=0-30%] to obtain the target compound (compound 1c, 1.13g, yield 46.86%) as a yellow solid, LCMS [M+1] + =180.
[0079] Step 2: Compound 1e (3-(3-(2-chloropyrimidin-4-yl)-1H-pyrrol-1-yl)-3-(cyanomethyl)azetidine-1-carboxylic acid tert Butyl ester) preparation
[0080] To a solution ...
Embodiment 2
[0088] Example 2 The General Method of Synthetic Compound 2 (TDM-180975)
[0089]
[0090] Step 1: Preparation of compound 2b (3-methyl-5-nitropyridinemethylamide)
[0091] A mixed solution of compound 2a (3 g, 18.4 mmol) and concentrated sulfuric acid (18 ml) was heated to 80° C. and stirred for 25 minutes. LCMS[M+H] + =182, the detection reaction is complete. Post-treatment: Cool the reaction solution to room temperature, pour it into ice water (100ml), then adjust the pH to neutral with sodium carbonate, extract the mixture three times with ethyl acetate (3*100ml), combine the organic phases, and wash with saturated brine , dried over anhydrous sodium sulfate, suction filtered, concentrated and pulled to dryness to obtain the yellow target compound (compound 2b, 3.33g, yield 94.2%), LCMS [M+1] + =182.
[0092] Step 2: Preparation of compound 2c (5-amino-3-methylpicolylamide)
[0093] Add palladium carbon (10%, 300 mg) to compound 2b (3.14 g, 17.33 mmol) in methanol ...
Embodiment 3
[0097] Example 3 The General Method of Synthetic Compound 3 (TDM-180976)
[0098]
[0099] Step 1: Preparation of compound 3b (ethyl 3-methyl-5-nitrolinoleate)
[0100] Concentrated sulfuric acid (40ml) was slowly added to ethanol (160ml) solution at 0°C, then compound 3a (4g, 24.5mmol) was added in batches to the reaction solution, and the reaction solution was heated to reflux and stirred for 72 hours. LCMS[M+H] + =211, the detection reaction is complete. Post-treatment: Cool the reaction solution to room temperature and pour it into water (50ml), extract three times with ethyl acetate (3*50mL), combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, suction filter, concentrate and extract Dry to obtain the yellow target compound (compound 3b, 3.73g, yield 72.56%), LCMS [M+1] + =211.
[0101] Step 2: Preparation of compound 3c (3-methyl-5-nitrolinoleic acid)
[0102] Add 1N sodium hydroxide (120ml, 120.16mmol) solution to compound 3b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com