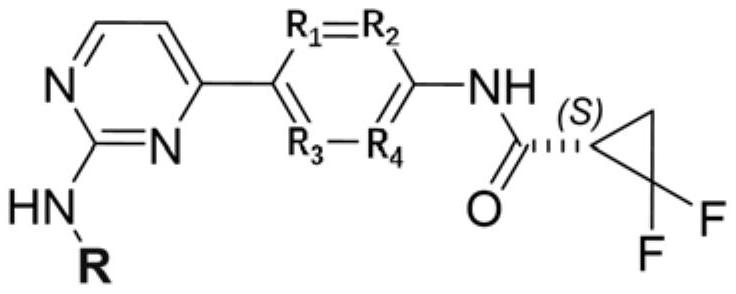
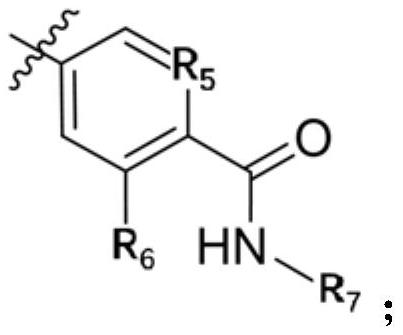
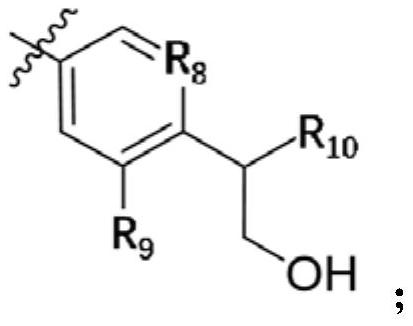
A kind of small molecular compound as jak kinase inhibitor and its use
A technology of small molecular compounds and compounds, applied in anti-inflammatory agents, organic chemistry, digestive system, etc., to achieve good JAK kinase activity and effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 The General Method for Synthesizing Compound 1 (TDM-180972)
[0076]
[0077] Step 1: Preparation of compound 1c (4-(2-chloropyrimidin-4-yl)aniline)
[0078] Add compound 1a (2g, 9.129mmol) to the three-necked flask, compound 1b (1.36g, 9.129mmol), tetrakistriphenylphosphine palladium (527g, 0.45mmol), potassium carbonate (2.5g, 18.258mmol), dioxane (20 mL) and water (20 mL), replaced with nitrogen several times, then the mixture was heated to 80° C. and stirred for 45 minutes. After the reaction, the reactant was concentrated under reduced pressure, and the residue was purified by silica gel chromatography (petroleum ether / ethyl acetate=0-60%) to obtain the target compound (compound 1c, 394 mg, yield 21%) as a pale yellow solid. LCMS[M+1] + =206.
[0079]Step 2: Preparation of compound 1e ((S)-N-(4-(2-chloropyrimidin-4-yl)phenyl)-2,2-difluorocyclopropane-1-carboxamide)
[0080] Compound 1c (300mg, 1.459mmol) and compound 1d (187mg, 1.531mmol) were adde...
Embodiment 2
[0084] Example 2 The General Method of Synthetic Compound 2 (TDM-180974)
[0085]
[0086] Step 1: Compound 2 ((S)-5-((4-(4-(4-(2,2-difluorocyclopropane-1-carboxamido)phenyl)pyrimidin-2-yl)amino)- 3-methylpyridoline) preparation
[0087] To a solution of compound 2a (80 mg, 0.258 mmol) in n-butanol (8 mL) was added compound 2b (78 mg, 0.517 mmol) and p-toluenesulfonic acid monohydrate (98 mg, 0.517 mmol), and the mixture was heated to 115° C. and stirred overnight . At the end of the reaction, the mixture was concentrated under reduced pressure, methanol was added to the residue, the solid was collected by filtration, and the solid was purified by preparative HPLC (formic acid) to obtain the white solid target compound TDM-180974 (compound 2, 18.9 mg, yield 10.6%). LCMS[M+H] + = 425.2.
[0088] 1 H NMR (400MHz, DMSO) δ10.73(s, 1H), 10.08(s, 1H), 8.89(d, J=2.2Hz, 1H), 8.60(d, J=5.3Hz, 1H), 8.23(d ,J=2.0Hz,1H),8.18(d,J=8.8Hz,2H),7.90(s,1H),7.79(d,J=8.8Hz,2H),7.49(d,J=5...
Embodiment 3
[0089] Example 3 The General Method of Synthetic Compound 3 (TDM-180977)
[0090]
[0091] Step 1: Preparation of compound 3b (4-amino-N-ethyl-2-methylbenzamide)
[0092] To a solution of compound 3a (1.8 g, 11.91 mmol) in N,N-dimethylformamide (80 mL) was added 2-(7-azabenzotriazole)-N,N,N',N'- Tetramethyluronium hexafluorophosphate (5.4g, 14.289mmol) and N,N-diisopropylethylamine (3.8g, 29.775mmol), the mixture was stirred for 5 minutes, then tetrahydrofuran (2M) of ethylamine was added ( 9 mL, 18 mmol) solution, and the mixture was stirred at room temperature overnight. The mixture was concentrated under reduced pressure to remove some solvent, water was added to the residue and extracted with ethyl acetate (100 mL*3), the organic phases were combined, washed with water (150 mL*3) and saturated brine (150 mL), dried over sodium sulfate, and extracted under reduced pressure The filtrate was concentrated and purified by silica gel chromatography (petroleum ether / ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com