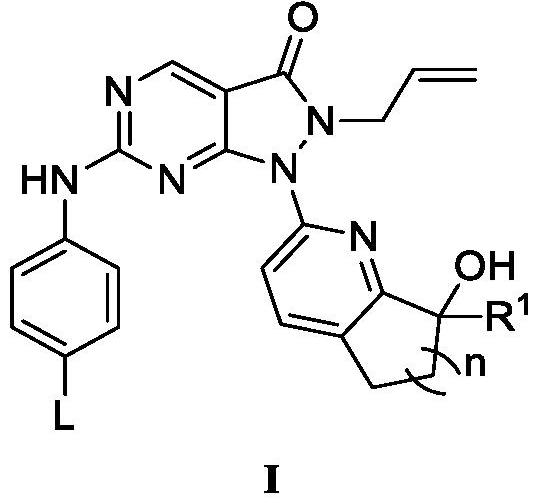
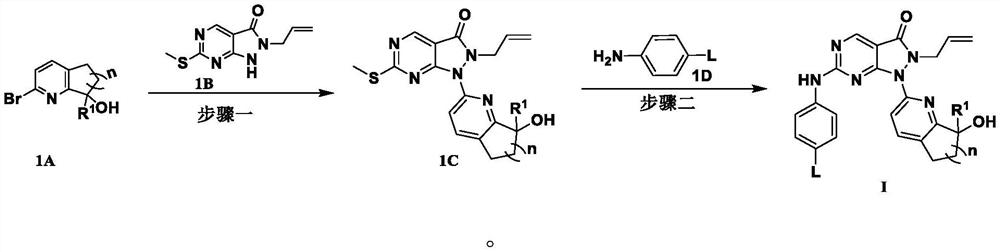
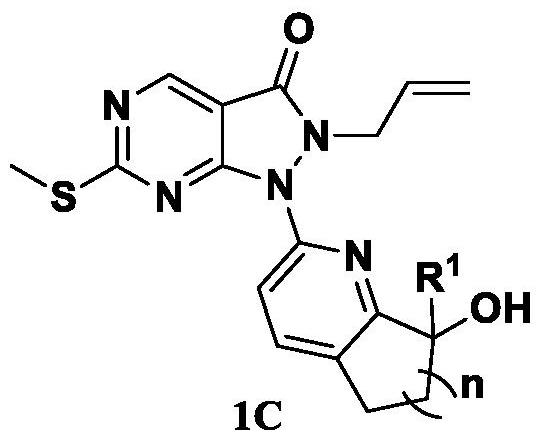
Pyrazolone-fused pyrimidine compound as well as preparation method and application thereof
A pyrazolone and pyrimidine technology, applied in the field of pyrazolone pyrimidine compounds, can solve problems such as single structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0399]
[0400] first step:
[0401] 1,2-Chloro-6,7-dihydro-5H-cyclopenta[b]pyridine (1 g, 6.51 mmol) (I-1-a) was added to 30 mL of toluene, followed by phosphorus oxybromide (4g, 13.95mmol), heated and stirred at 130°C for three days. After cooling and concentrating the reaction solution, slowly add saturated sodium bicarbonate solution until the pH value is approximately equal to 10, then extract three times with dichloromethane (3*30ml), dry over anhydrous sodium sulfate and concentrate to obtain a crude product, which is purified by column (acetic acid Ethyl ester: petroleum ether=0-15%), 2-bromo-6,7-dihydro-5H-cyclopentadiene [b] pyridine (I-1-b) 0.84g, brown solid, yield : 65%. LC-MS: m / z: (M+H) + =198.
[0402] Step two:
[0403] 2-Bromo-6,7-dihydro-5H-cyclopentadieno[b]pyridine (I-1-b) (0.8g, 4mmol) was added to 25ml of dichloromethane, and then 77% m-chloro Peroxybenzoic acid (1.34g, 6mmol) was also added to 25ml of dichloromethane, and this solution was adde...
Embodiment 2
[0413]
[0414] 2-allyl 1-(7-hydroxy-6,7-dihydro-5H-cyclopentadien[b]pyridin-2-yl)-6-(methylthio)-1,2-di Hydrogen-3H-pyrazolo[3,4d]pyrimidin-3-one (40mg, 0.11mmol) (I-1-g) and 3-chlorophenylperoxycarboxylic acid (30.2mg, 0.135mmol) were dissolved in 15ml In toluene, the resulting solution was stirred at room temperature for 2 h. 4-(4-Ethylpiperazin-1-yl)aniline (30mg, 0.14mmol) and N,N-diisopropylethylamine (29.0mg, 0.22mmol) were added to the above solution, and the reaction solution was heated at 90 °C under nitrogen protection and stirred for 16 hours. The reaction solution was concentrated, and the obtained solid was separated by thin-layer chromatography (dichloromethane:methanol=10:1) to obtain a crude product, which was then separated by preparative HPLC to obtain 2-allyl-6-((4-(4- Ethylpiperazin-1-yl)phenyl)amino)-1-(7-hydroxy-6,7-dihydro-5H-cyclopentadien[b]pyridin-2-yl)--1,2 -Dihydro-3H-pyrazolo[3,4-d]pyrimidin-3-one (I-2) 38.6 mg, white solid, yield 66.9%. 1H...
Embodiment 5
[0416]
[0417] first step:
[0418] Dissolve 67mg (0.25mmol) of N,N-dimethyl-1-(1-(4-nitrophenyl)piperidin-4-yl)methanamine (I-5-a) in 5ml of methanol under hydrogen , reacted for half an hour under palladium-carbon catalyzed conditions. The reaction solution was filtered and concentrated to obtain 56 mg of 4-(4-(dimethylamino)methyl)piperidin-1-yl)aniline (I-5-b) as a yellow solid with a yield of 97%. LC-MS: m / z: (M+H) + =234.
[0419] Step two:
[0420] 2-allyl-1-(7-hydroxyl 6,7-5H-cyclopenta[b]pyridin-2-yl)-6-(methylthio)-1,2-dihydro-3H-pyr Azo[3,4-d]pyridin-3-one (I-1-g) was dissolved in 10ml of toluene, 30mg (0.17mol, 77%) of 3-chloroperoxybenzoic acid was added, and the toluene was dissolved after stirring at room temperature for 15 minutes. Spin dry, then add 40mg (0.11mmol) 4-(4-(dimethylamino)methyl)piperidin-1-yl)aniline (I-5-b) dissolved in 5ml dimethyl sulfoxide, add 0.4ml trifluoroacetic acid, heated to 60°C and stirred for about 18 hours. The reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com