Chitosan-containing medicinal preparation, medicinal transdermal patch and preparation method of medicinal transdermal patch
A technology of pharmaceutical preparations and transdermal patches, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A1
[0096] (1) Prepare packaged medicine
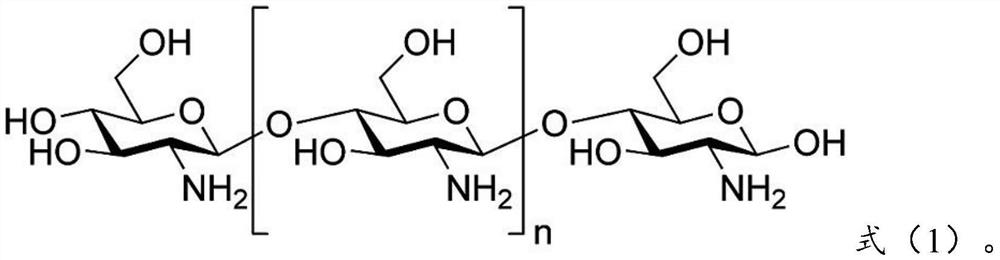
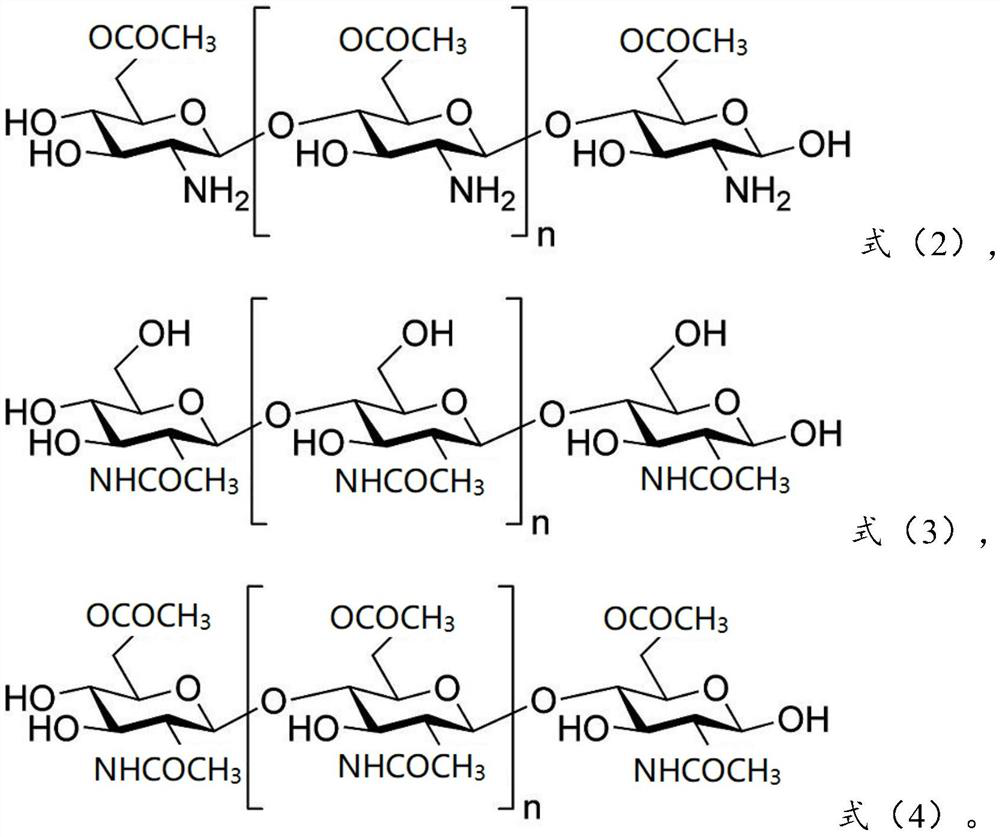
[0097] 0.28g olanzapine is dissolved in chitosan (molecular weight is 180,000) shown in 0.41g formula (1) and chitosan derivative shown in 0.21g formula (2) (molecular weight is 150,000, acylation degree is 1) in the aqueous solution to obtain the water phase; add the obtained water phase to 15 times the weight of the oil phase (which contains 0.2g emulsifier Tween-80, the rest is petroleum ether, the following are the same), ultrasonic 30min, forming W / The O-type emulsion is added with a cross-linking agent glutaraldehyde to carry out chemical cross-linking, and the obtained material is centrifuged and dried to obtain olanzapine-chitosan coatings with an average particle diameter of 387nm.
[0098] (2) Ingredients
[0099] Encapsulated drug: prepared in this embodiment;
[0100] Unpacked drug: olanzapine, 0.1g;
[0101] Transdermal absorption enhancer: isopropyl myristate, 0.57g;
[0102] Drug-loaded matrix: 7.17g in total, of which...
Embodiment A2
[0108] (1) Prepare packaged medicine
[0109] 0.25g olanzapine is dissolved in chitosan (molecular weight is 150,000) shown in 0.46g formula (1) and chitosan derivative shown in 0.19g formula (3) (molecular weight is 200,000, acylation degree is 1) into an aqueous solution to obtain a water phase; add the obtained water phase to 12 times the weight of the oil phase, and ultrasonicate for 30 minutes to form a W / O emulsion, then add a cross-linking agent glutaraldehyde for chemical cross-linking, and centrifuge the obtained material Separating and drying to obtain the olanzapine chitosan coating with an average particle diameter of 354nm.
[0110] (2) Ingredients
[0111] Encapsulated drug: prepared in this embodiment;
[0112] Unpacked drug: olanzapine, 0.1g;
[0113] Transdermal absorption enhancer: isopropyl myristate, 0.42g;
[0114] Drug-loading matrix: a total of 7.5g, of which 6.00g is 235A pressure-sensitive adhesive (containing carboxyl group), 0.75g is 2516 pressur...
Embodiment A3
[0120] 0.22g olanzapine is dissolved in chitosan (molecular weight is 200,000) shown in 0.41g formula (1) and chitosan derivative shown in 0.25g formula (4) (molecular weight is 180,000, acylation degree is 2) into an aqueous solution to obtain a water phase; add the obtained water phase to 10 times the weight of the oil phase, and ultrasonicate for 30 minutes to form a W / O emulsion, then add a cross-linking agent glutaraldehyde for chemical cross-linking, and centrifuge the obtained material Separating and drying to obtain the olanzapine chitosan coating with an average particle diameter of 436nm.
[0121] (2) Ingredients
[0122] Encapsulated drug: prepared in this embodiment;
[0123] Unpacked drug: olanzapine, 0.1g;
[0124] Transdermal absorption enhancer: Span 20, 0.7g;
[0125] Drug-loaded matrix: a total of 7.64g, of which 4.59g is 2852 pressure-sensitive adhesive (containing carboxyl group), and 3.06g is 2516 pressure-sensitive adhesive (containing hydroxyl group);...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com