Selenium-enriched probiotics for affecting mouse diarrhea and intestinal canal florae caused by irinotecan
A technology of selenium-enriched probiotics and irinotecan, which is applied in the field of microorganisms and can solve problems such as impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 Se-DD98
[0044] The Bifidobacterium longum DD98 strain (Bifidobacterium.longum DD98) used in the present invention, after extracting the bacterial genome DNA, is amplified using the 16S universal primer of the bacterium, and the 16SrDNA gene sequence of the bacterium is amplified, and the sequence is as shown in SEQ ID No: 1 shown.
[0045]By using 16SrDNA sequencing and BLAST analysis of nucleotide sequences, the strain is not completely identical to the corresponding sequences of all other Bifidobacterium longum known so far, but has certain homology. Through the analysis of sugar fermentation characteristics, compared with "Bergey's Bacteria Identification Manual", it is further confirmed that the strain belongs to Bifidobacterium longum, but it is different from the standard strain Bifidobacterium longum JCM 11342 (see Genebank number for its 16S rDNA sequence: LC306854. 1) The result of comparing the 16S rDNA sequence shows that th...
Embodiment 2
[0053] Example 2 Changes in body weight of mice
[0054] The mice were randomly divided into 4 groups, 12 in each group, which were normal control group, CPT-11 group, Se-DD98 low-dose group, and Se-DD98 high-dose group. The normal control group and the CPT-11 group were intragastrically administered distilled water, the Se-DD98 low-dose group was intragastrically administered bacterial powder (the number of viable bacteria was 1×108 CFU / kg), and the Se-DD98 high-dose group was intragastrically administered bacterial powder (the number of viable bacteria was 1× 109 CFU / kg), 0.2 ml / 10g, 1 time / day. Establish irinotecan diarrhea model. From day 0 to day 24, the mice in each group were intraperitoneally injected with sterile water or bacterial solution; on day 18, the normal control group was intraperitoneally injected with sterile saline, and each mouse in the other groups was intraperitoneally injected with 150 mg / kg CPT- 11. The injection volume is 0.1 ml / 10 g; on the 17th d...
Embodiment 3
[0064] Example 3 Incidence of diarrhea in mice and evaluation of diarrhea grade
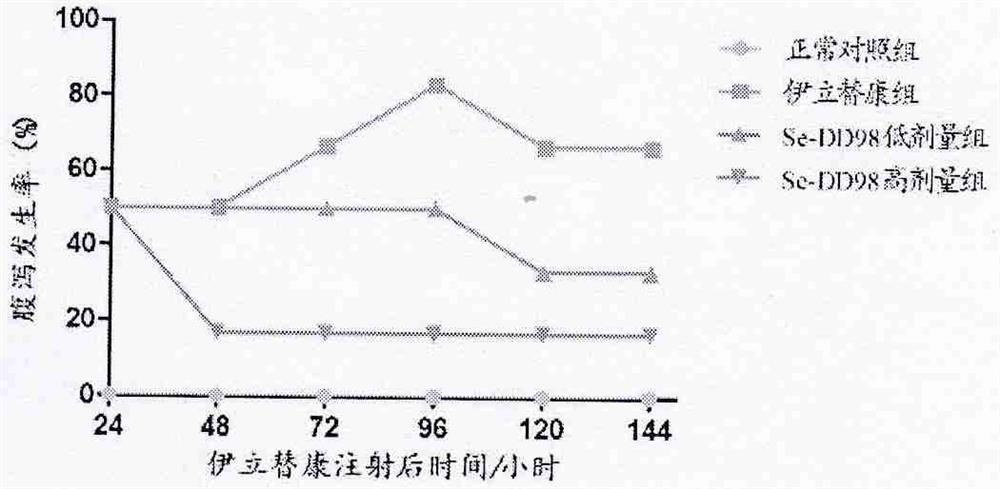
[0065] After 24 hours of irinotecan injection, the mice in each group were observed for diarrhea and scored at 48, 72, 96, 120 and 144 hours, respectively.
[0066] Table 4 Evaluation criteria for diarrhea in mice
[0067]
[0068] Table 5 Effect of Se-DD98 on the average diarrhea grade of mice with irinotecan-induced diarrhea
[0069]
[0070] Table Note: Compared with the normal group, *p△ p△△ p<0.01.
[0071] in conclusion:
[0072] 1. figure 2 The results showed that the incidence of diarrhea in the irinotecan group and the Se-DD98 low-dose group changed over time, showing a trend of increasing gradually at first, then decreasing and then remaining unchanged. The Se-DD98 high-dose group remained unchanged after 48 hours of decline, but the incidence of diarrhea in the Se-DD98 high-dose and low-dose groups was lower than that of the irinotecan group;
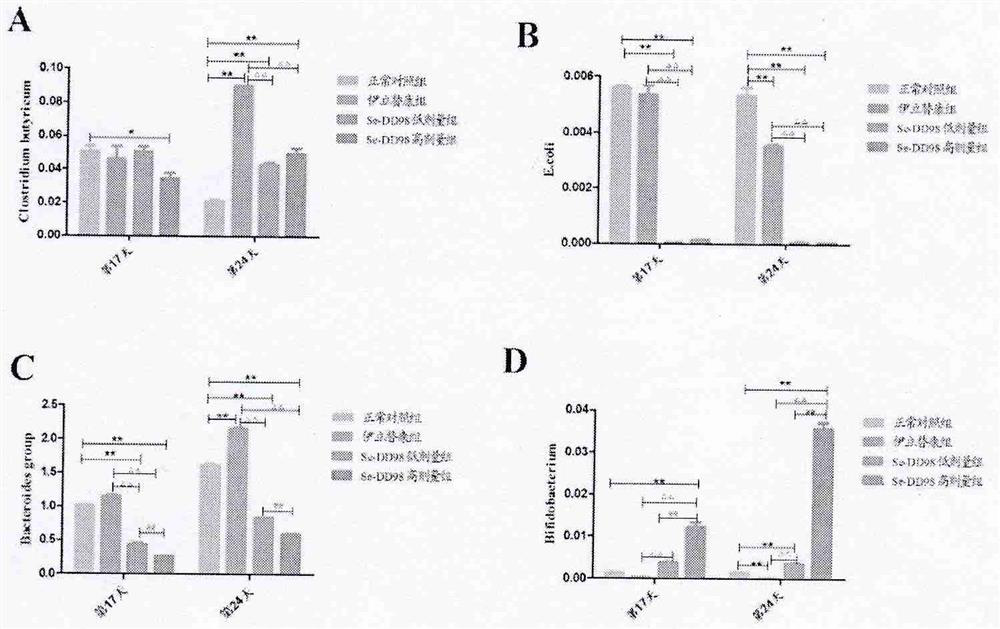
[0073] 2. The results in Tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com