Method for preparing nitrobenzene through catalysis of polyoxometallate
A polyoxometalate and catalytic preparation technology, which is applied in the field of polyoxometalate catalytic preparation of nitrobenzene, can solve problems such as environmental pollution, corrosion of reaction equipment and the like, achieve simple requirements for reaction equipment, reduce pollution, and mild reaction conditions controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
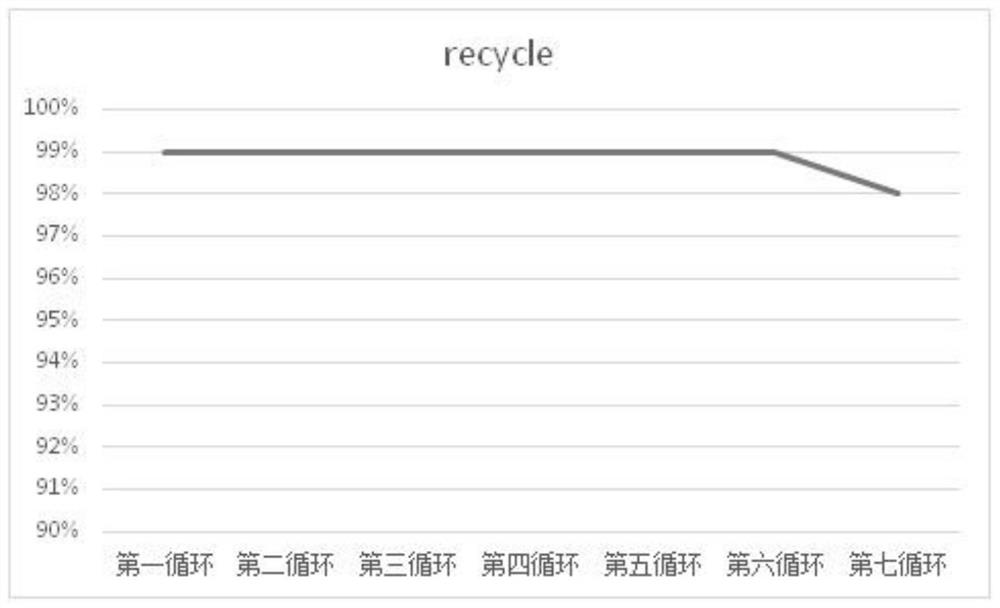
Image
Examples
Embodiment 1
[0030] Embodiments of the present invention provide a method for preparing nitrobenzene by polyoxometalate catalysis, comprising the following steps:
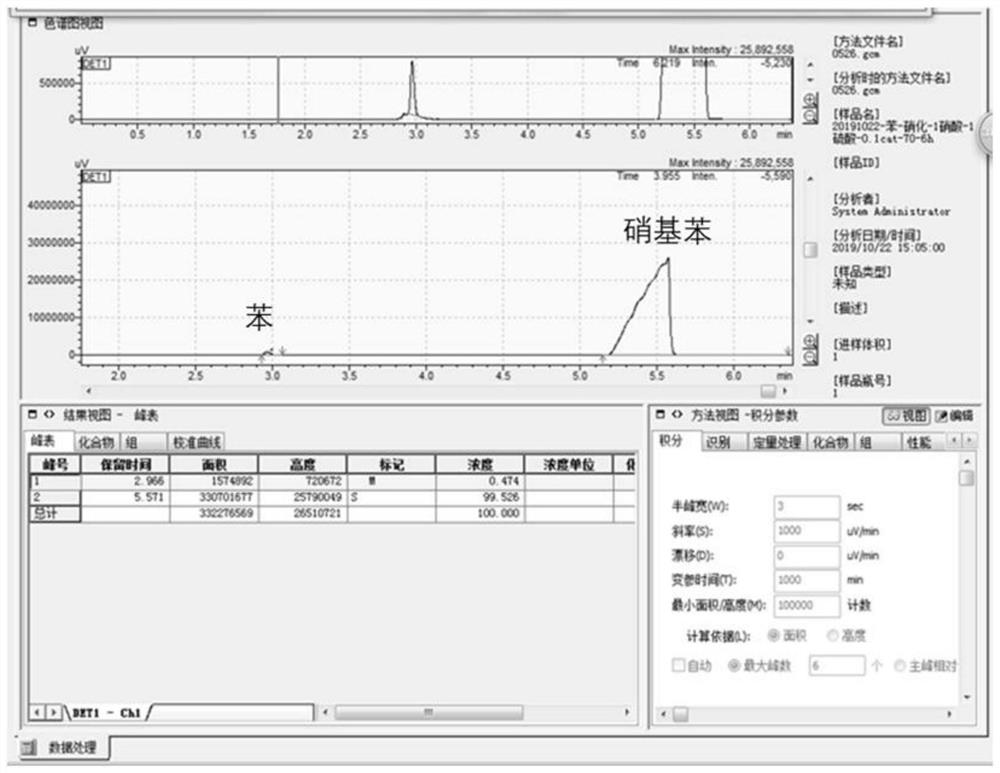
[0031] a. Add 10ml of benzene, 1.6g of phosphotungstic acid polyoxometalate with Anderson structure, and 7.7mL of concentrated nitric acid with a concentration of 70% into the reaction kettle in turn, control the reaction temperature at 60 degrees, and react for 6 hours;
[0032] b. After the nitration reaction is finished, the liquid should be layered to obtain an organic phase and an aqueous phase, and the aqueous phase should be separated and removed after liquid separation; 20ml of water is added to the organic phase to wash off unreacted acid, and the aqueous phase should be separated and removed after liquid separation;
[0033] c. Add 20g of anhydrous sodium sulfate to the organic phase, filter out the desiccant after drying, the yellow organic phase liquid is the crude product of nitrobenzene, the crude product of nitrob...
Embodiment 2
[0035] Embodiments of the present invention provide a method for preparing nitrobenzene by polyoxometalate catalysis, comprising the following steps:
[0036] a. Add 10ml of benzene, 1.7g of silicotungstomolybdate polyoxometalate with Anderson structure, 7.7mL of concentrated nitric acid with a concentration of 70% and 0.64ml of concentrated sulfuric acid with a concentration of 98% into the reaction kettle in sequence, and control the reaction temperature to 80 degree, react for 2 hours;
[0037] b. After the nitration reaction is finished, the liquid should be layered to obtain an organic phase and an aqueous phase, and the aqueous phase should be separated and removed after liquid separation; 20ml of water is added to the organic phase to wash off unreacted acid, and the aqueous phase should be separated and removed after liquid separation;
[0038] c. Add 20g of anhydrous sodium sulfate to the organic phase, filter out the desiccant after drying, the yellow organic phase l...
Embodiment 3
[0040] Embodiments of the present invention provide a method for preparing nitrobenzene by polyoxometalate catalysis, comprising the following steps:
[0041] a. 10ml of benzene, 1.7g of borotungstomolybdate polyoxometalate with Anderson structure, 8.47mL of concentrated nitric acid with a concentration of 70% and 1.28ml of concentrated sulfuric acid with a concentration of 98% were successively added to the reaction kettle, and the reaction temperature was controlled to be 60 degree, react for 6 hours;
[0042] b. After the nitration reaction is finished, the liquid should be layered to obtain an organic phase and an aqueous phase, and the aqueous phase should be separated and removed after liquid separation; 20ml of water is added to the organic phase to wash off unreacted acid, and the aqueous phase should be separated and removed after liquid separation;
[0043] c. Add 20g of anhydrous calcium chloride to the organic phase, filter out the desiccant after drying, the yello...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com