Targeted chitosan nano-silver gel as well as preparation method and application thereof
A chitosan nano, silver gel technology, applied in pharmaceutical formulations, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc., to achieve broad application prospects, avoid drug resistance, dispersibility and stability good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
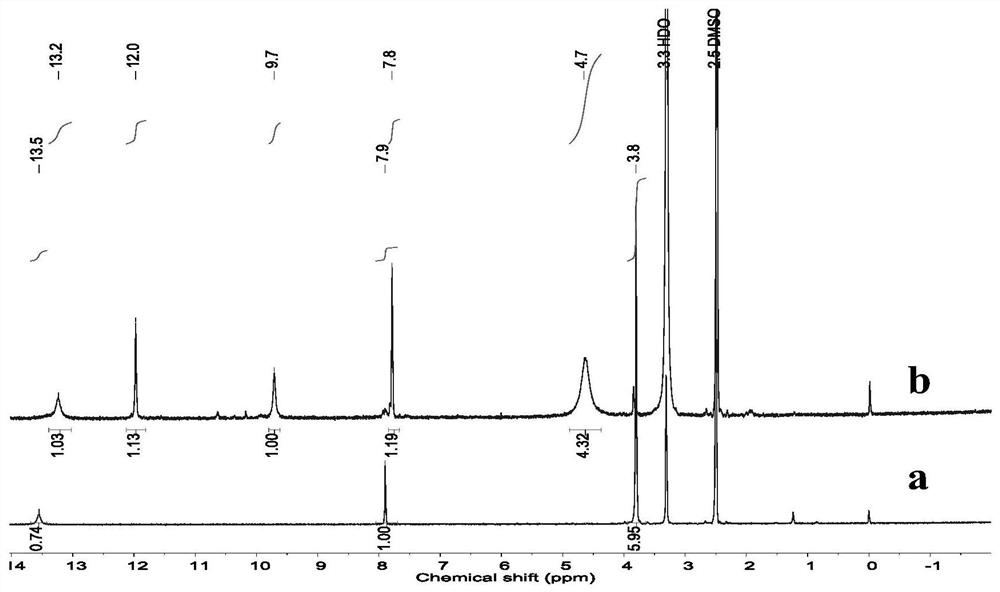
[0038] Put 0.5g of dimethyl imidazole-4,5-dicarboxylate into a 100mL three-neck round bottom flask, add 10mL of methanol, and heat to reflux at 70°C until the dimethyl imidazole-4,5-dicarboxylate is completely dissolved Afterwards, 2 mL of 80% hydrazine hydrate was added and refluxed for 3 h, washed with methanol, centrifuged twice, and the obtained sample was vacuum-dried to obtain the product imidazole-4,5-dicarboxylic acid dimethylhydrazide. Such as figure 1 Shown is imidazole-4,5-dicarboxylic acid dicarboxylhydrazide 1 HNMR spectrum. Dimethyl imidazole-4,5-dicarboxylate ( figure 1 a): δ3.8 (CH3), δ7.9 (CH), δ13.5 (NH). Imidazole-4,5-dicarboxylic acid dicarboxylic acid hydrazide ( figure 1 b): After hydrazinolysis, the proton resonance peak of the methoxy group δ3.8 disappears, the new resonance peaks of δ9.7 and δ12.0 are attributed to the NH peak of the hydrazide group, and δ4.7 is assigned to the NH2 peak of the hydrazide group , indicating that the hydrazinolysis w...
Embodiment 2
[0040] Weigh 1.7760 g of chitosan (MW=5KDa), place it in a 250 mL three-necked round bottom flask, dissolve it in 50 mL of NaAc / HAc buffer solution with pH=4.5, and ultrasonicate for 30 min to dissolve it. Weigh 0.2246g of sodium periodate, dissolve it in 20mL of NaAc / HAc buffer solution with pH=4.5, place the two solutions in an ice bath, fill with nitrogen gas, degas for 20 minutes, mix them, and stir them in an ice bath for 24 hours Afterwards, 10 mL of ethylene glycol was added to terminate the reaction. Take out the product solution, pour it into a petri dish, put it into a refrigerator to freeze at -20°C, take out the solidified sample and place it in a freeze dryer to freeze-dry to obtain the product formyl chitosan.
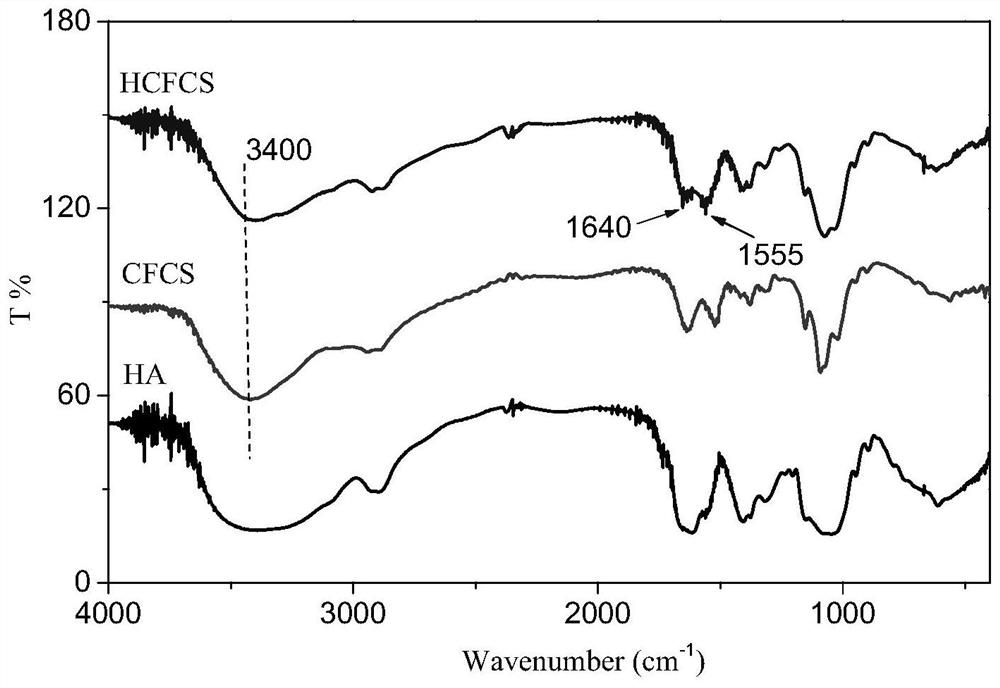
[0041] Weigh 0.05g of formyl chitosan in a single-necked flask and add 20mL of deionized water to dissolve it, dissolve 0.0055g of imidazole-4,5-dicarboxylic acid diformylhydrazide with 10mL of dimethyl sulfoxide, and add formyl chitosan to the Add imidazo...
Embodiment 3
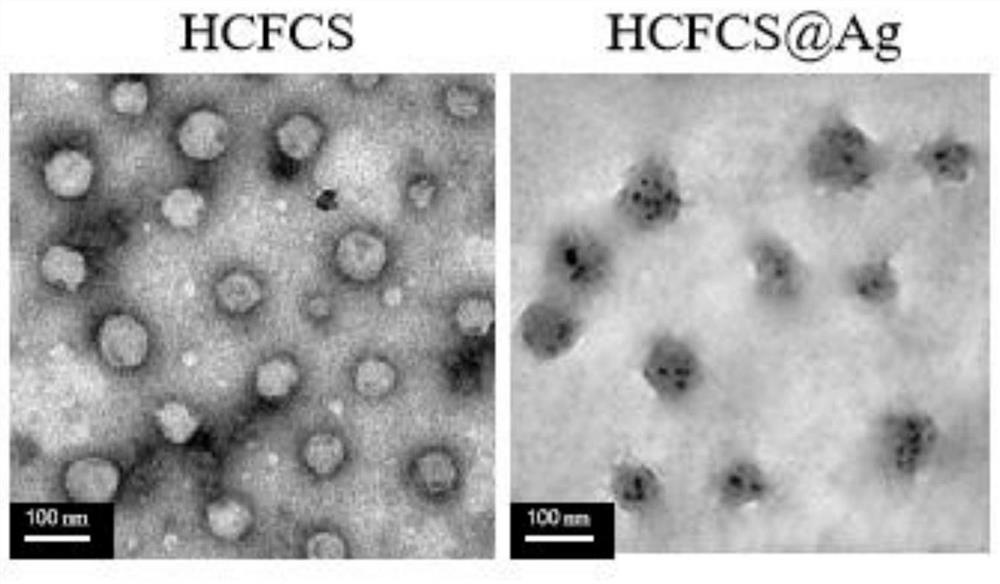
[0043] Weigh 15mg of formyl chitosan (MW=5kDa) in 30mL of deionized water, heat and ultrasonicate in a water bath at 70°C, until it is completely dissolved, slowly add 5mL of 0.6mg / mL imidazole-4,5-dicarboxylate dropwise Acid dimethyl hydrazide solution, after ultrasonication for 20 minutes, add 2 mL of 1.7 mg / mL silver nitrate solution dropwise to the reaction solution, continue ultrasonication for 10 minutes, adjust the pH value to 8-9 with sodium hydroxide solution, and add 10% boron dropwise Sodium hydride solution 0.5mL, then dropwise add 1% hyaluronic acid solution with a volume ratio of 1:1 to imidazole cross-linked chitosan gel (CFCS), and continue ultrasonication for 20min, dialyze the product solution (MWCO=2000kDa), freeze Dry to obtain targeting nano-silver gel (HCFCS@Ag). Such as image 3 Shown are the TEM images of the targeted chitosan nanogel (HCFCS) prepared in Example 2 and the targeted nanosilver gel (HCFCS@Ag) prepared in this example. The particle size d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com