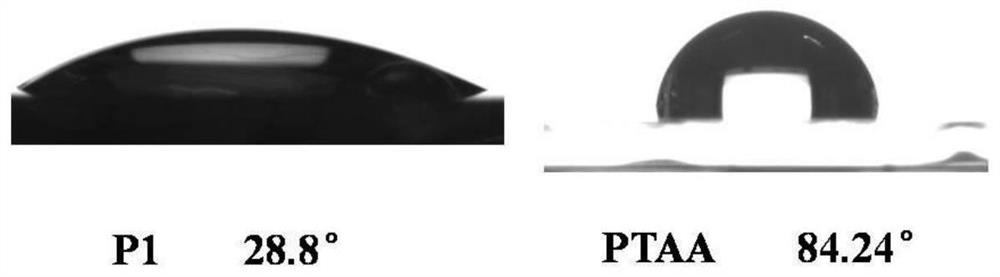
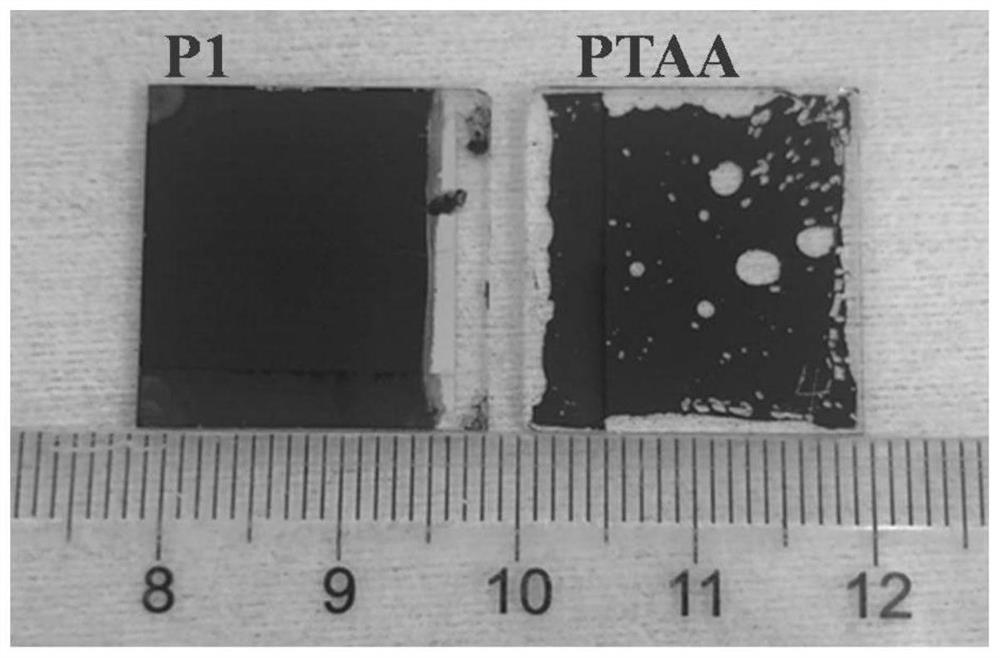
Hole Transport Polymers Containing Benzyl Alcohol Groups and Their Applications
A technology of hole transport and polymer, which is applied in the field of perovskite solar cells, can solve the problems of poor wettability of perovskite precursor, limitation of large-area device preparation, and incomplete coverage of perovskite layer, etc., to achieve easy large-scale preparation , Conducive to the effect of effective extraction and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Synthesis of polymer P1:
[0071] Under argon, 0.1078g (0.25mmol) of compound 5, 0.1739g (0.25mmol) of compound 8, 0.46mg of Pd(OAc) 2 , 1.64mg of phosphine ligand Sphos, 5.00mg of phase transfer catalyst Aliquat 336 and 5mL of anhydrous toluene were mixed and stirred. After heating to 95°C, an oxygen-free potassium carbonate aqueous solution (2M, 0.8mL) was added, and then stirred at 95°C for 5 hours. Add 15.00 mg of phenylboronic acid and react for 5 hours and 0.50 mL of bromobenzene for 5 hours. Cool down to 80°C and add 1.00 g of sodium diethyldithiocarbamate, and continue stirring for 24 hours. After the reaction was completed, it was extracted with dichloromethane, washed with water three times, and dried over anhydrous sodium sulfate. After filtration and concentration, the product was settled into 150 mL of methanol. The resulting solid, 12.58 mg (0.33 mmol) of sodium borohydride and 60 mL of dichloromethane were stirred at reflux under an argon atmosphere fo...
Embodiment 2
[0074] Synthesis of polymer P2:
[0075] Under argon, 0.1644g (0.25mmol) of compound 6, 0.1278g (0.25mmol) of compound 10, 0.46mg of Pd(OAc) 2 , 1.64mg of phosphine ligand Sphos, 5.00mg of phase transfer catalyst aliquat 336 and 5mL of anhydrous toluene were mixed and stirred, and after the temperature was raised to 95°C, an oxygen-free potassium carbonate aqueous solution (2M, 0.8mL) was added, then stirred at 95°C for 5 hours, and then sequentially Add 15.00 mg of phenylboronic acid for 5 hours and 0.50 mL of bromobenzene for 5 hours. Cool down to 80°C, add 1.00 g of sodium diethyldithiocarbamate, and continue stirring for 24 hours. After the reaction was completed, it was extracted with dichloromethane, washed with water three times, and dried over anhydrous sodium sulfate. Filter, concentrate, and settle the product into 150 mL of methanol. The resulting solid, 12.58 mg (0.33 mmol) of sodium borohydride and 60 mL of dichloromethane were stirred at reflux under an argon ...
Embodiment 3
[0078] Synthesis of polymer P3:
[0079] Under argon, 0.1644g (0.25mmol) of compound 6, 0.1313g (0.25mmol) of compound 9, 0.46mg of Pd(OAc) 2 , 1.64mg of phosphine ligand Sphos, 5.00mg of phase transfer catalyst aliquat 336 and 5mL of anhydrous toluene were mixed and stirred, and after the temperature was raised to 95°C, an oxygen-free potassium carbonate aqueous solution (2M, 0.8mL) was added, then stirred at 95°C for 5 hours, and then sequentially Add 15.00 mg of phenylboronic acid for 5 hours and 0.50 mL of bromobenzene for 5 hours. Cool down to 80°C and add 1.00 g of sodium diethyldithiocarbamate, and continue stirring for 24 hours. After the reaction was completed, it was extracted with dichloromethane, washed with water three times, and dried over anhydrous sodium sulfate. After filtration and concentration, the product was settled into 150 mL of methanol. The resulting solid, 12.58 mg (0.33 mmol) of sodium borohydride and 60 mL of dichloromethane were stirred at refl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com