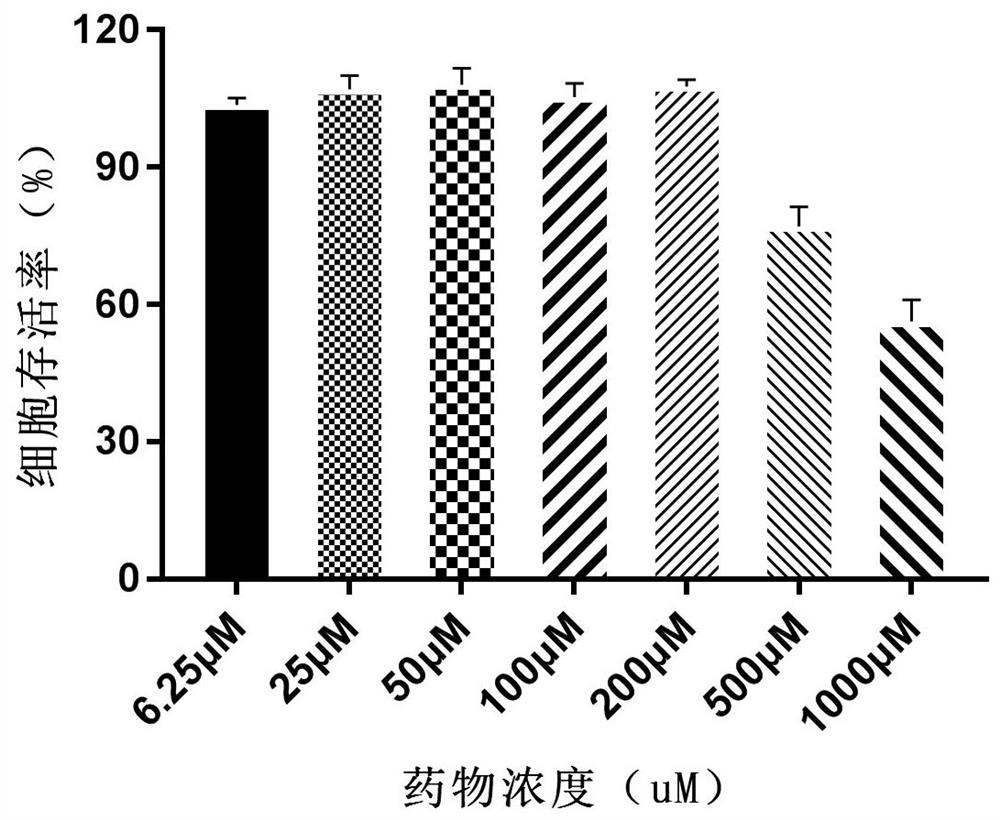
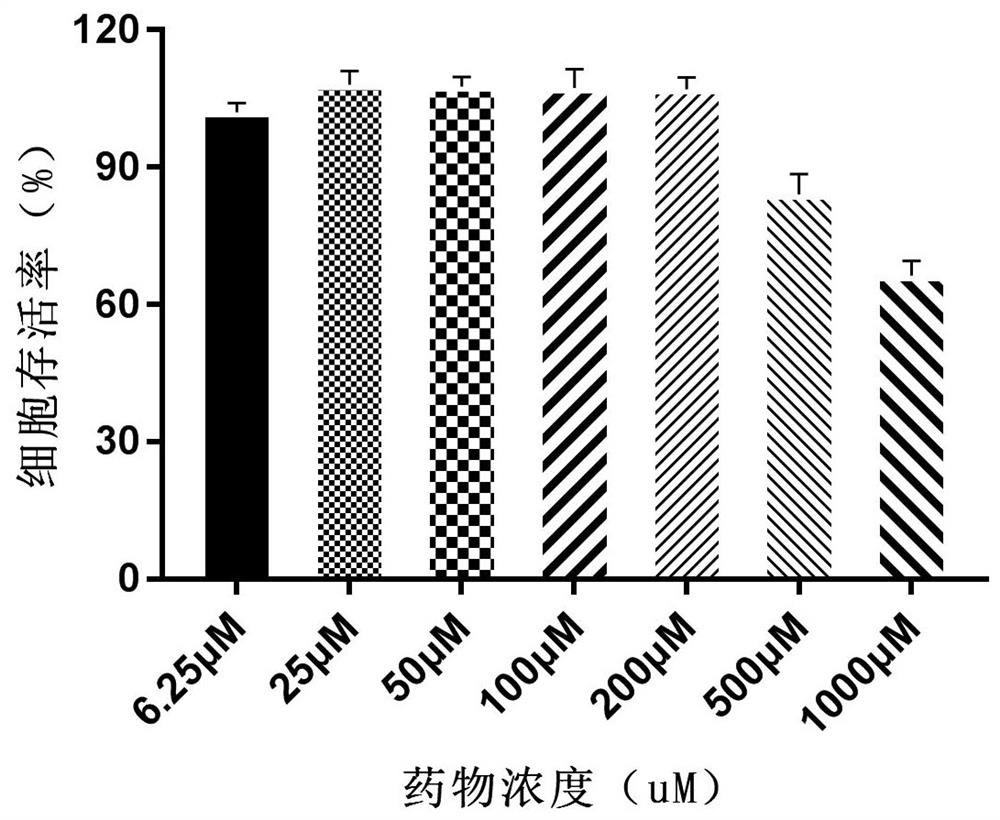
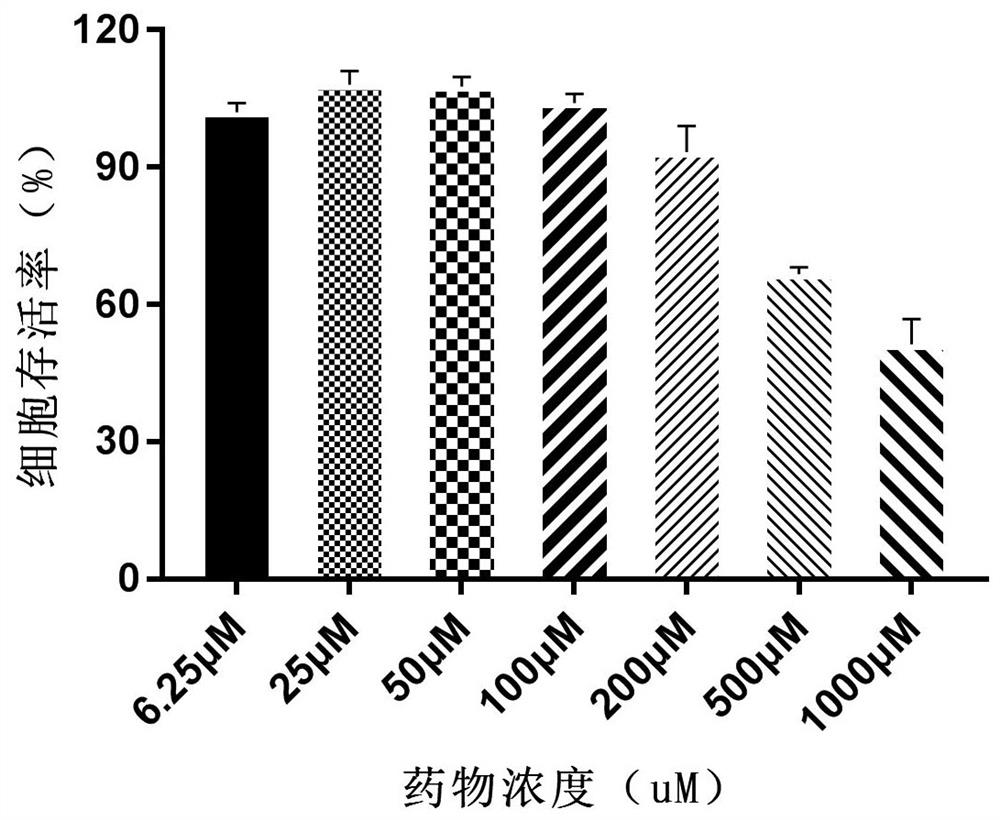
Alpha-configuration nucleoside and application in treating cat coronavirus infection
A configuration, nucleoside technology, applied in the treatment of coronavirus infection in cats or other animals, α-configuration nucleosides and corresponding prodrugs, and the field of solvates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of Example 1 Compound 1
[0038]
[0039] Under nitrogen protection, compound 1A (25.16 g, 45.53 mmol, 1.0 eq) was dissolved in anhydrous dichloromethane (55 mL, 2.1 times), stirred at 0°C, and TfOH (8.1 mL, 91.06 mmol, 2.0 eq), after the dropwise addition was completed, stir for 10 minutes. Then TMSOTf (17.3 mL, 95.61 mmol, 2.1 eq) was slowly added dropwise. After the dropwise addition was completed, the reaction was stirred at this temperature for 30 minutes. Slowly add TMSCN (22.8 mL, 182.11 mmol, 4.0 eq) dropwise, and after the dropwise addition, stir below 0°C for 2 hours. TLC detected that the reaction of the raw materials was complete, and slowly added 22 mL of triethylamine dropwise. After the drop, the reaction solution rose to room temperature, then added sodium bicarbonate (34.42 g), added dropwise 120 mL of water, stirred for 10 minutes after the drop, and separated the liquid , collected the organic phase, extracted the aqueous phase with dic...
Embodiment 2
[0044] Synthesis of Example 2 Compound 2
[0045]
[0046]Compound 1 (0.5 g, 1.72 mmol, 1.0 eq) was suspended in 10 mL of pyridine, DMAP (21 mg, 0.17 mmol, 0.1 eq) was added, and isobutyric anhydride (870 mg, 5.49 mmol, 3.2 eq ). After the dropwise addition, stir overnight at room temperature, TLC shows that the reaction of the raw materials is complete, spin the reaction solution to dryness, add 20 mL of dichloromethane to dissolve, add 20 mL of saturated sodium bicarbonate solution and stir for 10 minutes, separate the liquids, and use dichloromethane for the aqueous phase Extraction (20 mL×1), combined organic phases, dried over anhydrous sodium sulfate, filtered, spin-dried, and column chromatography gave compound 2 as a white solid (689 mg, yield: 79.9%).
[0047] 1H NMR (500 MHz, DMSO-d6) : 8.03 (s, 1H), 7.93 – 7.81 (m, 2H), 6.93(d, J = 3.5 Hz, 1H), 6.72 (d, J = 3.5 Hz, 1H) , 4.95 (s, 1H), 4.53 (s, 1H),4.12 (s, 1H), 3.96 – 3.94 (m, 1H), 3.77 – 3.75 (m, 1H), 2.63 – 2...
Embodiment 3
[0049] Synthesis of Example 3 Compound 3
[0050]
[0051] Compound 1 (0.5 g, 1.72 mmol, 1.0 eq) was suspended in 10 mL of pyridine, TMSCl (653 mg, 6.01 mmol, 3.5 eq) was slowly added dropwise, and stirred at room temperature for 1 hour. Then octanoyl chloride (307 mg, 1.89 mmol, 1.1 eq) was slowly added dropwise, and stirred at room temperature until TLC showed that the reaction of the starting material was complete. Spin the reaction solution to dryness, add 20 mL of dichloromethane to dissolve, add 20 mL of saturated sodium bicarbonate solution and stir for 10 minutes, separate the layers, extract the aqueous phase with dichloromethane (20 mL×1), combine the organic phases, and anhydrous sulfuric acid Sodium-dried, filtered, spin-dried, and column chromatography gave compound 2 as a white solid (480 mg, yield: 67.0%).
[0052] 1H NMR (500 MHz, DMSO-d6): 8.32 (br, 1H), 8.13 (s, 1H), 7.02 (d, J =3.5 Hz, 1H), 6.81 (d, J = 3.5 Hz, 1H), 5.50 (d, J = 4.5 Hz, 1H), 5.30 (d, J ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com