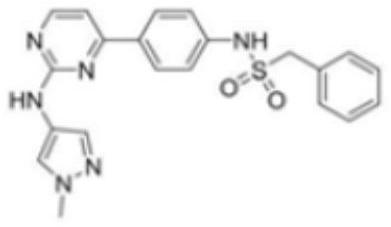
Pharmaceutical compound used as JAK kinase inhibitor
A drug compound and compound technology, which can be used in drug combinations, antipyretics, anti-inflammatory agents, etc., can solve problems such as few reports of Tyk2, and achieve the best therapeutic effect, good JAK kinase activity and the effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 General method for synthesis of compound 1 (TDM-180944)
[0049]
[0050] Step 1: Preparation of compound 1 (TDM-180944)
[0051] To a solution of compound 1a (60 mg, 0.225 mmol) in pyridine (5 mL) was added compound 1b (65.8 mg, 0.315 mmol) at room temperature, and the mixture was heated to 70° C. and stirred for 6 h. Then the mixture was concentrated under reduced pressure, and the residue was purified by silica gel chromatography (dichloromethane: 10% methanol in dichloromethane = 70:30) and formic acid preparation to obtain yellow solid compound 1, TDM-180944, namely 1- (2-Fluorophenyl)-N-(4-(2-(((1-methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)phenyl)methanesulfonamide (34.5mg , 34.97% yield).
[0052] 1 H NMR(400MHz, DMSO-d 6 )δ10.34(s,1H),9.45(s,1H),8.45(d,J=5.2Hz,1H), 8.10(d,J=8.6Hz,2H),7.93(s,1H),7.55( s, 1H), 7.45–7.30 (m, 4H), 7.25–7.17 (m, 3H), 4.60 (s, 2H), 3.84 (s, 3H). LCMS[M+1] + = 439.2.
Embodiment 2
[0053] Example 2 General method for synthesis of compound 2 (TDM-180945)
[0054] Prepare compound 2 in a similar manner to Example 1: TDM-180945, namely 1-(3-fluorophenyl)-N-(4-(2-(((1-methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)phenyl ) Methanesulfonamide (26.1 mg, 19.8% yield).
[0055] 1 H NMR(400MHz, DMSO-d 6 )δ10.25(s,1H),9.45(s,1H),8.45(d,J=5.2Hz,1H), 8.12(d,J=8.6Hz,2H),7.93(s,1H),7.56( s, 1H), 7.40 (dd, J = 14.3, 7.6 Hz, 1H), 7.32 (d, J = 8.6 Hz, 2H), 7.26-7.15 (m, 2H), 7.11 (d, J = 7.3 Hz, 2H ), 4.63 (s, 2H), 3.84 (s, 3H). LCMS[M+1] + = 439.2.
Embodiment 3
[0056] Example 3 General method for synthesis of compound 3 (TDM-180958)
[0057]
[0058] Step 1: Preparation of compound 3c (3-cyanobenzylaminothiocarbamate)
[0059] Compound 3b (690 mg, 9.08 mmol) was added to the ethanol (13 mL) solution of compound 3a (1.78 g, 9.08 mmol), the reaction solution was heated to 80° C. and stirred for 1 hour, LCMS [M+H] + =192, the detection reaction is complete. Post-treatment: the reaction solution was concentrated to dryness to obtain the white target compound (compound 3c, 1.7 g, yield 97.7%), LCMS [M+1] + =192.
[0060] Step 2: Preparation of compound 3d ((3-cyanophenyl)methanesulfonyl chloride)
[0061] To the N-chlorosuccinimide (4.85g, 36.32mmol) in acetonitrile (20mL) was added 2N hydrochloric acid solution (2.5mL) and compound 3c (1.74g, 9.08mmol), the reaction solution was stirred at room temperature for 30 minutes . Post-treatment: After the reaction was completed, the acetonitrile was concentrated and removed, water (15 mL) was added, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com