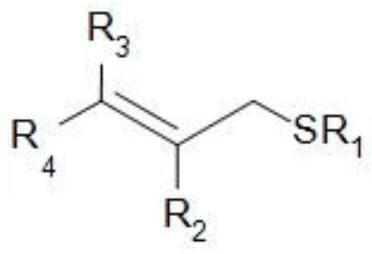
Allyl sulphide compound and preparation method thereof
A technology of allyl sulfide and compounds, which is applied in the field of allyl sulfide compounds and their preparation, can solve the problems of reduced scope of application of substrates, metal residues in products, unfavorable practical operations, etc., and achieves a simple and efficient synthesis method , short reaction route, less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Get α-methylstyrene (24mg, 0.2mmol), N-p-tolylthiosuccinimide (53mg, 0.24mmol), pyridine hydrochloride (11mg, 0.1mmol) and join in a 10mL reaction flask, and then Add 2 mL of 1,2-dichloroethane, heat to 60°C, and stir for 12 hours. After the reaction, add 10 mL of ethyl acetate, add 5 mL of water for extraction, dry the organic phase with anhydrous sodium sulfate, and obtain The target compound was 42 mg, and the yield of the target compound was measured to be 88%.
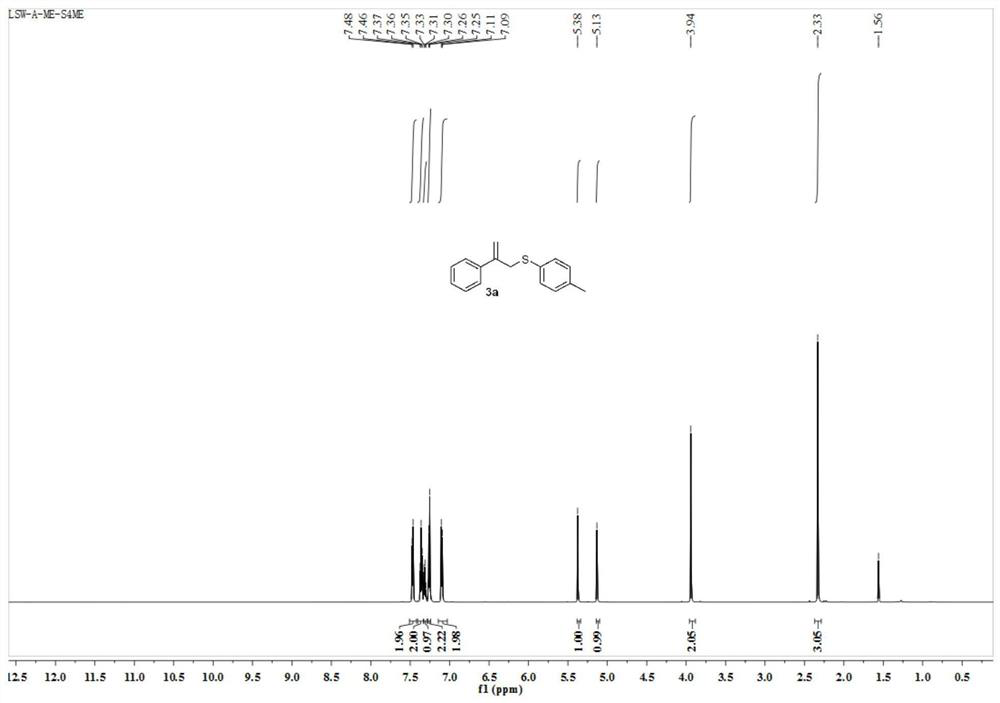
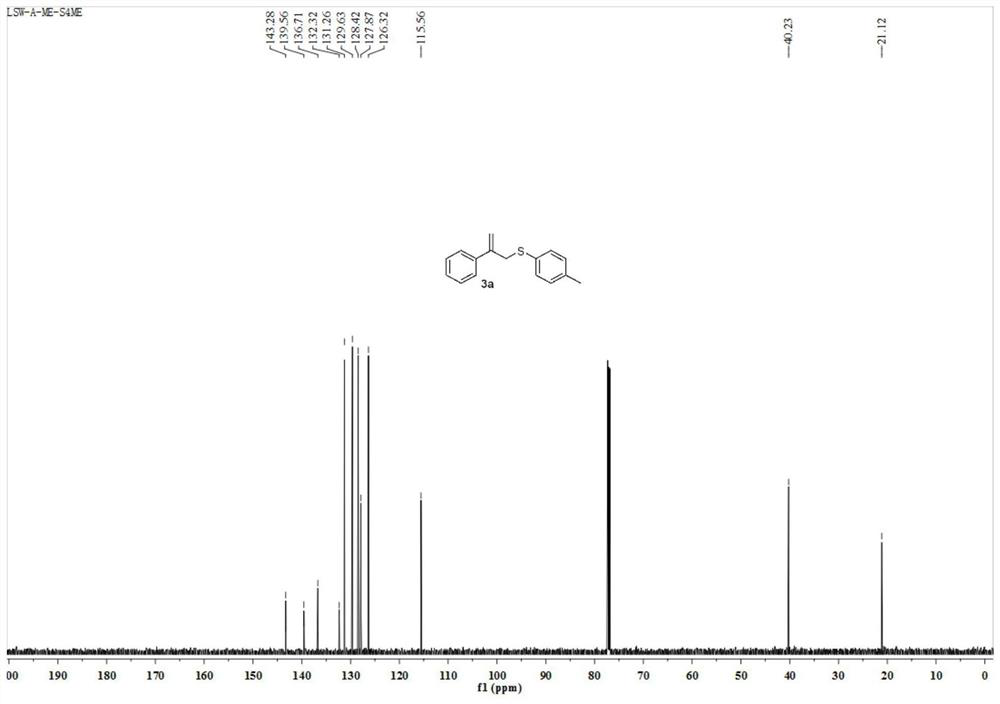
[0036] Such as Figure 1 ~ Figure 2 Shown, the target compound of present embodiment 1 is 2-phenyl-3-(4-methylphenyl) allyl sulfide, and the structural formula of target compound is:
[0037]
[0038] The proton nuclear magnetic resonance spectrum data of target compound are as follows: 1H NMR (600MHz, CDCl3) δ7.47(d, J=7.3Hz, 2H), 7.36(t, J=7.5Hz, 2H), 7.31(t, J=7.2Hz, 1H), 7.26(d, J =7.0Hz, 2H), 7.10(d, J=7.9Hz, 2H), 5.38(s, 1H), 5.13(s, 1H), 3.94(s, 2H), 2.33(s, 3H).
[0039] The carbon nuclear ma...
Embodiment 2
[0041] On the basis of Example 1, the impact of the molar ratio of terminal olefins, N-tolylthiosuccinimide, and pyridine hydrochloride on the yield of the target compound was studied, and the raw materials were weighed according to the data in Table 1, Test according to the method of embodiment 1.
[0042] Table 1
[0043]
[0044] As can be seen from the data in table 1, when N-p-tolylthiosuccinimide consumption is little, substrate α-methylstyrene can not react fully, and when excessive (1.2 times), productive rate improves to some extent, but far When the excess (3 times) is used, the yield will no longer increase. In order to save raw materials, an excess of 1.2 times is selected. Pyridine hydrochloride has the best effect when the catalytic amount (0.5 times) is used, and the amount of 0.2 times is not enough to efficiently catalyze the reaction. When the amount is far excessive (3 times), the reaction yield will no longer increase, and the excess hydrochloric acid m...
Embodiment 3
[0046] Take α-methylstyrene (24mg, 0.2mmol), N-p-chlorophenylthiosuccinimide (58mg, 0.24mmol), and pyridine hydrochloride (11mg, 0.1mmol) into a 10mL reaction flask, Add 2 mL of 1,2-dichloroethane, heat to 60°C, and stir for 12 hours. After the reaction, add 10 mL of ethyl acetate, add 5 mL of water for extraction, dry the organic phase with anhydrous sodium sulfate, and perform column chromatography. 46 mg of the target compound was obtained, and the yield of the target compound was measured to be 90%.
[0047] The target compound of this embodiment is 2-phenyl-3-(4-chlorophenyl) allyl sulfide, and the structural formula of the target compound is:
[0048]
[0049] The proton nuclear magnetic resonance spectrum data of target compound are as follows: 1 H NMR (600MHz, CDCl 3 )δ7.44–7.43(m,2H),7.38–7.34(m,2H),7.32(d,J=7.2Hz,1H),7.24(d,J=0.9Hz,4H), 5.38(s,1H ),5.14(s,1H),3.94(s,2H).
[0050] The carbon nuclear magnetic resonance spectrum data of target compound are as fol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com