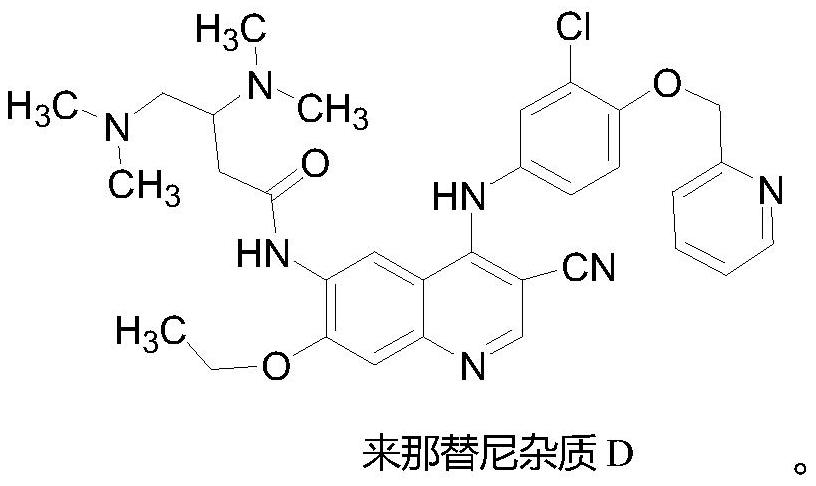
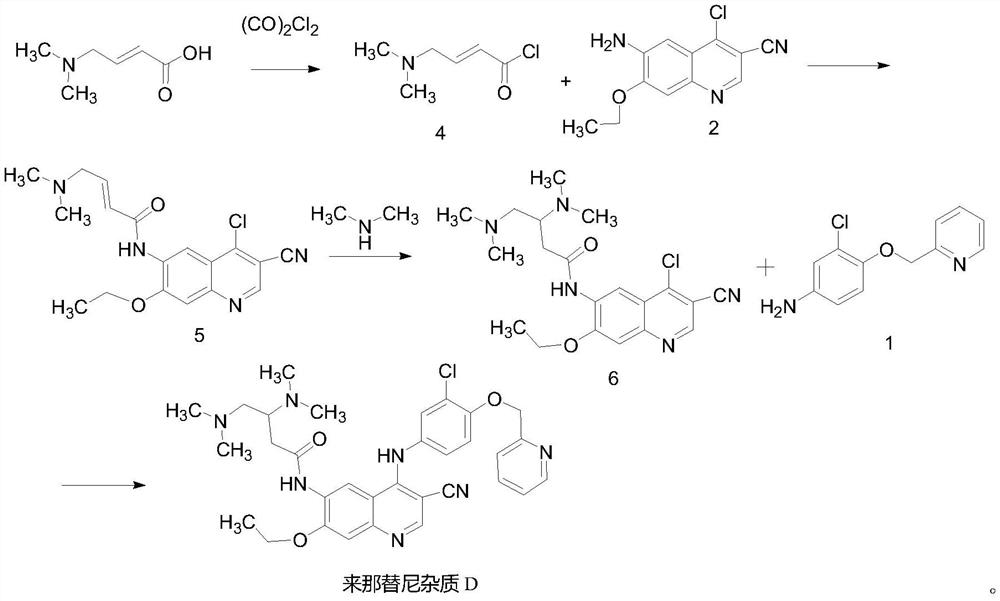
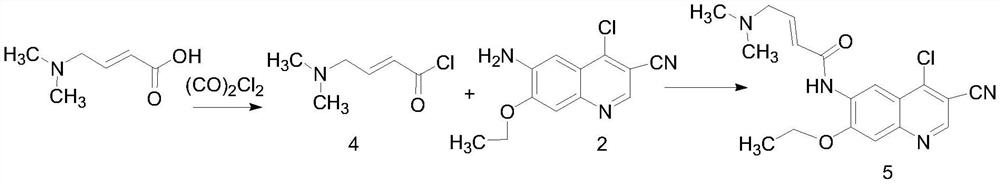
A kind of preparation method of neratinib impurity d
A neratinib and impurity technology, which is applied in the field of neratinib impurities, can solve the problems of low yield of target product, many reaction by-products, and the need for column separation of products, so as to achieve high yield of final product and high yield of by-products. The effect of less and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of compound 5
[0025]
[0026] Add 3.3g (20mmol) of N,N-dimethylaminotrans-croton hydrochloride into a 250mL dry three-necked flask, add 50mL of anhydrous THF, 0.2mL of anhydrous DMF, and cool down to about 0°C in a low-temperature cooling tank , Measure 1.66 mL (20 mmol) of oxalyl chloride with a syringe, dilute it with 15 mL of anhydrous THF and slowly add it dropwise to the system. The addition is completed in 10 minutes, and the reaction is carried out at room temperature for 3 hours to obtain compound 4. Cool down to 0°C, add 2.5g (10mmol) of compound 2 in 50mL of N-methylpyrrolidone (NMP) solution dropwise, drop it over 20min, move to room temperature for reaction, monitor the reaction progress by TLC, and end the reaction after 4h. Add 1M potassium carbonate solution to adjust the pH to about 11, filter under reduced pressure, wash the filter cake with water, and then recrystallize and dry the filter cake with THF / water to obtain 2.76 g of solid (...
Embodiment 2
[0028] Preparation of compound 6
[0029]
[0030] Put 2.76 g of the above-mentioned product (compound 5) into the reaction kettle, add 20 mL (2mol / L, 20 mmol) dimethylamine tetrahydrofuran solution, 40 mL THF, seal the reaction in an oil bath at 100 ° C for 8 hours, and add additional dimethylamine tetrahydrofuran solution 10mL (2mol / L, 20mmol), continued to react for 8h, the raw material reaction was completed, the reaction was terminated, the solvent was concentrated under reduced pressure to remove the crude product, and then recrystallized and dried with THF / water to obtain 2.2g of solid (compound 6), the yield was 71%. ESI-MS(m / z):404[M+H] + .
Embodiment 3
[0032] Preparation of neratinib impurity D
[0033]
[0034] Add 1.17g (5mmol) of compound 1 and 2g (5mmol) of compound 6 into a dry 100mL reaction flask, add 50mL of acetonitrile, 0.15g (0.5mmol) of zinc nitrate, heat up to the reflux reaction of the system under mechanical stirring, and a large amount of A yellow solid was formed, and TLC detected (PE / EA=1 / 4) that the reaction of the raw material was basically complete, and the reaction was terminated. The temperature of the system was lowered to room temperature, filtered, and the filter cake was washed with acetonitrile and ethanol respectively, and the filter cake was added to 40 mL of potassium carbonate solution with a concentration of 1M. After stirring, it was suction-filtered under reduced pressure, and the filter cake was washed with 50 mL of water several times. The filter cake was recrystallized and dried with acetonitrile / THF to obtain 2.7 yellow solids (neratinib impurity D), with an HPLC purity of 99.4% and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com