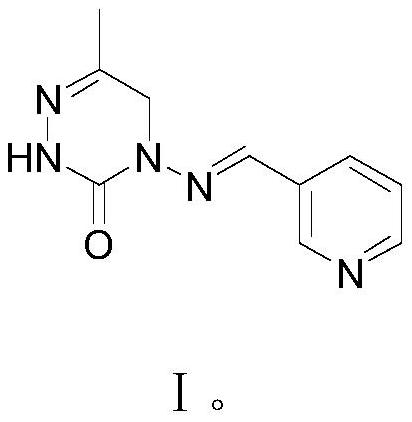
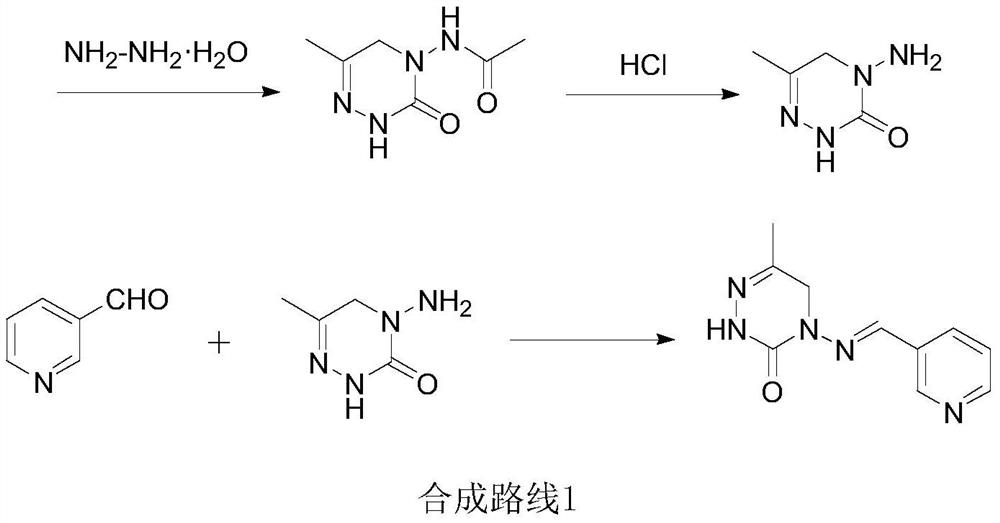
A kind of preparation method of pymetrozine
A technology of pymetrozine and monochloroacetone, which is applied in the field of pesticide chemistry, can solve the problems of large amount of three wastes, low product purity, and many side reactions, and achieve reduced polymer generation, high yield and purity, and reduced by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the preparation of carbohydrazide (Ⅲ)
[0056] In a 1000 ml four-neck flask connected with a stirring, thermometer, and reflux condenser, add 200 g of methanol, 200 g of water, and 90.1 g (1.0 mole) of dimethyl carbonate (II 1 ), 275.0 grams (2.2 moles) of 40% hydrazine hydrate, heated, stirred and reacted at 70 to 75°C for 3 hours, cooled to 20-25°C, filtered, the filter cake was washed with 20 grams of water, and dried to obtain 88.7 grams of carbohydrazide (Ⅲ), the yield is 98.5%, and the liquid phase purity is 99.9%.
Embodiment 2
[0057] Embodiment 2: the preparation of carbohydrazide (Ⅲ)
[0058] Into the 1000 ml four-neck flask connected with stirring, thermometer and reflux condenser, add 200 g of ethanol, 200 g of water, 118.1 g (1.0 mole) of diethyl carbonate (II 2 ), 275.0 grams (2.2 moles) of 40% hydrazine hydrate, heated, stirred and reacted at 75 to 80°C for 2 hours, cooled to 20-25°C, filtered, the filter cake was washed with 20 grams of water, and dried to obtain 88.9 grams of carbohydrazide (Ⅲ), the yield is 98.8%, and the liquid phase purity is 99.9%.
Embodiment 3
[0059] Embodiment 3: Preparation of pyridin-3-yl methylene carbohydrazide (IV)
[0060] In the 1000 milliliter four-neck flask that is connected with stirring, thermometer, constant pressure dropping funnel and reflux condenser, add 200 grams of methyl alcohol, 100 grams of water, 45.0 grams (0.5 mole) carbohydrazide (Ⅲ) obtained in embodiment 1 , heated, kept between 55 and 60°C, added dropwise a mixed solution of 53.0 grams (0.5 moles) of 3-formylpyridine and 100 grams of methanol, and the addition was completed in 3 hours, then stirred and reacted at 60 to 65°C for 6 hours, and cooled to 20-25°C, filter, wash the filter cake with 30 g of water, and dry to obtain 88.8 g of pyridin-3-ylmethylenecarbohydrazide (IV), with a yield of 99.2% and a liquid phase purity of 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com