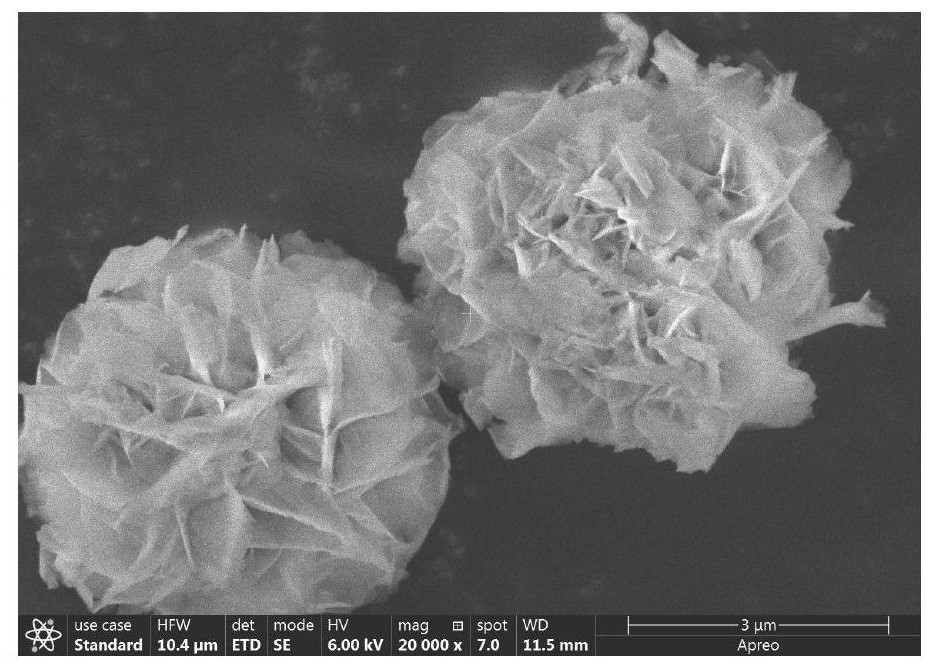
Nanometer lithium-rich manganese-based positive electrode material and precursor, base material and preparation method thereof
A lithium-rich manganese-based, cathode material technology, applied in the direction of positive electrode, nanotechnology, nanotechnology, etc., can solve the problems of crystal structure change, stability deterioration, first charge and discharge efficiency deterioration, etc., to reduce crystal structure changes. , Improve the electrochemical performance, avoid the effect of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment includes the following steps:
[0034]Step (1), use nickel sulfate hexahydrate, cobalt sulfate heptahydrate, and manganese sulfate monohydrate to prepare metal salt mixed solution A according to the molar ratio of Ni: Co: Mn 0.2: 0.2: 0.6, and the total concentration of nickel, cobalt, and manganese metal ions is 2.0 mol / L. The preparation concentration is the sodium hydroxide aqueous solution B of 10mol / L, and the preparation concentration is the ammonia solution C of 12mol / L. Dissolve an appropriate amount of sodium oleate in deionized water, ultrasonically disperse, and prepare solution D with a mass fraction of 0.01%;
[0035] Step (2), pour 1 / 4 volume of sodium oleate solution D into the 50L reactor, control the stirring rate to 400rpm, and the temperature of the reactor to 60°C. Subsequently, pass through ammonia solution C and sodium hydroxide solution B, adjust the pH to 12, and control the ammonia concentration to 6-6.5g / L.
[0036] Step (3),...
Embodiment 2
[0044] This embodiment includes the following steps:
[0045] Step (1), use nickel sulfate hexahydrate, cobalt sulfate heptahydrate, and manganese sulfate monohydrate to prepare metal salt mixed solution A according to the molar ratio of Ni: Co: Mn 0.15: 0.15: 0.7, the total concentration of nickel, cobalt, and manganese metal ions is 2.0mol / L. Prepare potassium hydroxide aqueous solution B with a concentration of 8 mol / L, and prepare ammonia solution C with a concentration of 12 mol / L. Dissolve an appropriate amount of sodium oleate in deionized water, ultrasonically disperse, and prepare a solution D with a concentration of 0.02%;
[0046] Step (2), pour 1 / 3 volume of sodium oleate solution D into the 50L reactor, control the stirring rate to 500rpm, and the temperature of the reactor to 65°C. Subsequently, pass through ammonia solution C and potassium hydroxide solution B, adjust the pH to 11.5, and control the ammonia concentration to 7-7.5g / L.
[0047] Step (3), add so...
Embodiment 3
[0052] This embodiment includes the following steps:
[0053] Step (1), use nickel sulfate hexahydrate, cobalt sulfate heptahydrate, and manganese sulfate monohydrate to prepare metal salt mixed solution A according to the molar ratio of Ni: Co: Mn 0.2: 0.2: 0.6, and the total concentration of nickel, cobalt, and manganese metal ions is 2.0 mol / L. Prepare potassium hydroxide aqueous solution B with a concentration of 8 mol / L, and prepare ammonia solution C with a concentration of 8 mol / L. Dissolve an appropriate amount of potassium oleate in deionized water, ultrasonically disperse, and prepare solution D with a mass concentration of 0.01%;
[0054] Step (2), pour 1 / 3 volume of potassium oleate solution D into the 50L reactor, control the stirring rate to 600rpm, and the temperature of the reactor to 60°C. Subsequently, pass through ammonia solution C and potassium hydroxide solution B, adjust the pH to 11.8, and control the ammonia concentration to 8-8.5g / L.
[0055] Step ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com