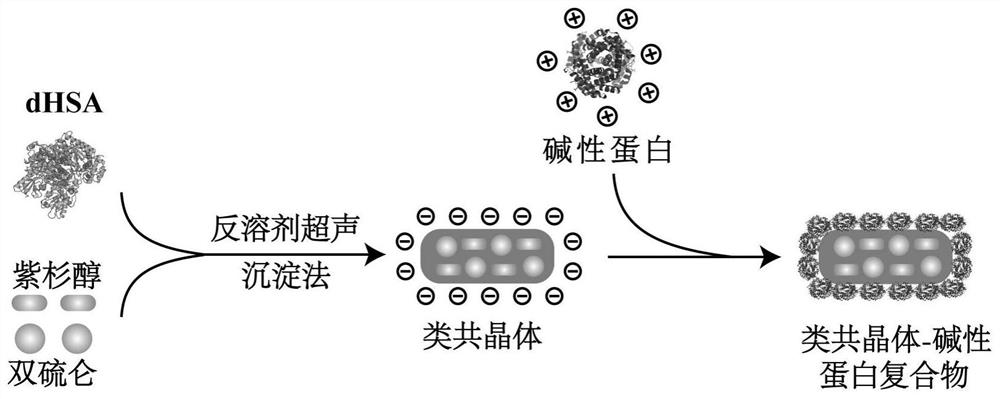
A kind of co-crystal-like drug compound of basic protein and its preparation method and application
A technology of co-crystals and complexes is applied in the field of preparation and application of co-crystal-like-basic protein drug complexes, which can solve the phenomenon of tumor cell multidrug resistance, chemotherapy failure, and inability to effectively reverse tumor multidrug resistance. and other problems, to achieve the effect of solving multidrug resistance, high drug loading and simple composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0069] Example 1: Formulation optimization of paclitaxel-disulfiram co-crystals
[0070] preparation process such as figure 1 As shown, the formulation optimization process is as follows:
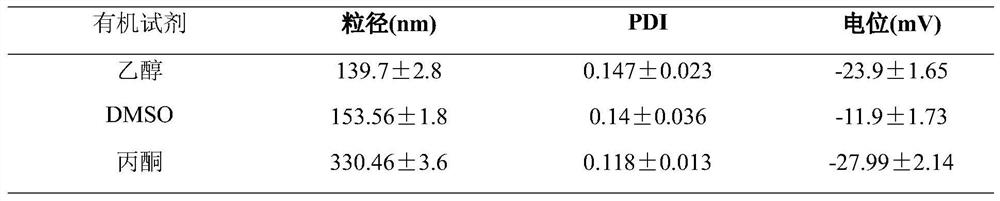
[0071] (1) Effects of organic reagents on particle size and potential of paclitaxel-disulfiram co-crystals
[0072] Accurately weigh three groups of 5 mg of paclitaxel and 1 mg of disulfiram API and place them in EP tubes, respectively add 0.5 ml of ethanol / acetone / DMSO for ultrasonic dissolution as the organic phase; take 10 ml of dHSA solution as the aqueous phase and place them in a vial. , placed in an ice bath on ice for 5 minutes; under stirring (1500 r / min), the organic phase was added dropwise to the aqueous phase, continued stirring for 30 seconds, transferred to the probe for ultrasound, and 230W power ultrasound for 15 minutes under ice bath conditions , and the residual organic solvent was removed by evaporation under reduced pressure to obtain paclitaxel-disulfiram hybrid nan...
example 2
[0080] Example 2: Preparation of paclitaxel-disulfiram co-crystals
[0081] dHSA (1mg / ml) 10ml
[0082] Paclitaxel 10mg
[0083] Disulfiram 2mg
[0084] Ethanol 0.5ml
[0085] The preparation process is as follows:
[0086] Accurately weigh 10 mg of paclitaxel and 2 mg of disulfiram in a centrifuge tube, add 0.5 ml of ethanol for ultrasonic dissolution and use as the organic phase; take 10 ml of 1 mg / ml dHSA solution as the aqueous phase and place it in a vial, and place it on ice to pre-cool. 5 minutes; under stirring (1500 r / min), the organic phase was added dropwise to the aqueous phase, and after continuing to stir for 30 seconds, it was transferred to the probe for ultrasound, and 230W power was ultrasound for 15 minutes under ice bath conditions. Ethanol was removed to obtain paclitaxel-disulfiram co-crystals.
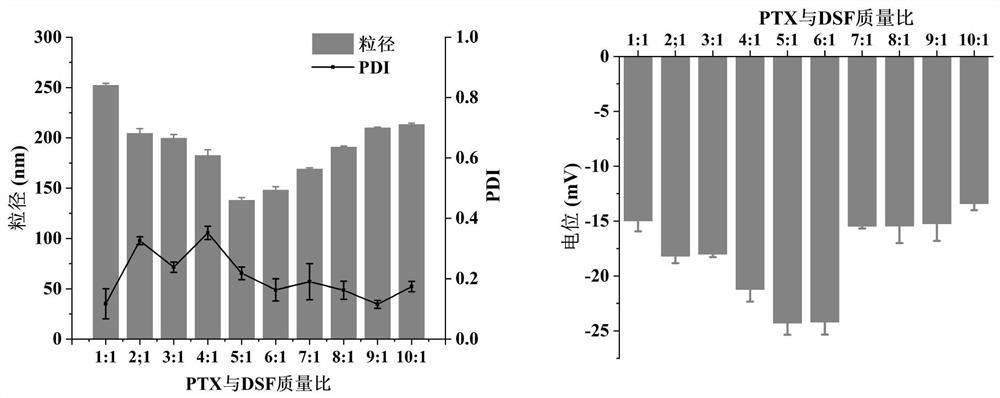
[0087] The obtained paclitaxel-disulfiram co-crystals were measured for particle size and potential, and the particle size results are shown in Figure 5 ,...
example 3
[0089] Example 3: Determination of Encapsulation Efficiency and Drug Loading Capacity of Paclitaxel-Disulfiram Co-crystals
[0090] In order to investigate the drug loading and encapsulation efficiency of the eutectic, the free drug in the preparation was separated by ultrafiltration and centrifugation, and the content of the drug in the nanoparticles was determined by high performance liquid chromatography. The specific steps are as follows: Precisely measure 1.5 ml of the eutectic-like crystal in Example 2 into an ultrafiltration tube, add 1.5 ml of equal volume of purified water and mix, centrifuge at 3500 rpm for 30 min, and take 20 μL of ultrafiltrate for injection , using high performance liquid chromatography to determine the content of free PTX and DSF, namely Wfree; take another 1ml of the same batch of eutectic, add 5ml methanol to break the demulsification, sonicate for 20min, after cooling to room temperature, add methanol to the mark, After filtration through a 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com