Autophagy-based stem cell myocardial cell induced differentiation method and application thereof
A technology for inducing differentiation and cardiomyocytes, applied in the field of biomedicine, can solve the problems of complicated and cumbersome operations, unstable differentiation efficiency, time-consuming and expensive, etc., and achieve the effects of improving efficiency, improving induction and differentiation efficiency, and shortening induction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
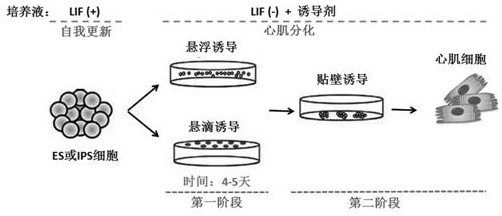
[0040] refer to figure 1 , a method for inducing differentiation of cardiomyocytes from stem cells based on autophagy, comprising the following steps:
[0041] Step 1, preparation of autophagy inducer:
[0042] Rapamycin was dissolved in DMSO, prepared as a 200µM stock solution, and stored at -20°C. Rapamycin was added to the culture medium when the cells were treated to make the final concentration 20nM, and the solvent DMSO (0.1‰) was used as a control, and it was added every time the medium was changed.
[0043] Step 2, stem cell culture: Mouse embryonic stem cells were inoculated on a culture dish treated with 0.1% Gelatin, containing 15% fetal bovine serum, 2 mM non-essential amino acids, 0.1 mM β-mercaptoethanol, 10 4 U / ml mouse LIF Medium, 100 U / ml penicillin and streptomycin in DMEM high-glucose medium, at 37°C, the volume fraction is 5% CO 2 cultured in a cell culture incubator.
[0044] Step 3. Stem cell induction (first stage): Take embryonic stem cells in loga...
Embodiment 2
[0049] refer to figure 1 , a method for inducing differentiation of cardiomyocytes from stem cells based on autophagy, comprising the following steps:
[0050] Step 1, preparation of autophagy inducer:
[0051] KU0063794 was dissolved in DMSO, prepared as a 10mM stock solution, and stored at -20°C. KU0063794 was added to the culture medium to make the final concentration of 1µM when the cells were treated, and the solvent DMSO (0.1‰) was used as a control, and was added every time the medium was changed.
[0052] Step 2, stem cell culture: Mouse embryonic stem cells were inoculated on a culture dish treated with 0.1% Gelatin, containing 15% fetal bovine serum, 2 mM non-essential amino acids, 0.1 mM β-mercaptoethanol, 10 4 U / ml mouse LIF Medium, 100 U / ml penicillin and streptomycin in DMEM high-glucose medium, at 37°C, the volume fraction is 5% CO 2 cultured in a cell culture incubator.
[0053] Step 3. Stem cell induction (first stage): Take embryonic stem cells in logari...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com