Amphiphilic benzoxazine and polyethylene glycol modified organic silicon resin and preparation method and application thereof
A polyethylene glycol modification and benzoxazine technology, which is applied in coatings, biocide-containing paints, antifouling/underwater coatings, etc., can solve the problems of high cost and biological hazards, and avoid application costs High, excellent antifouling performance, effect of improving antifouling performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
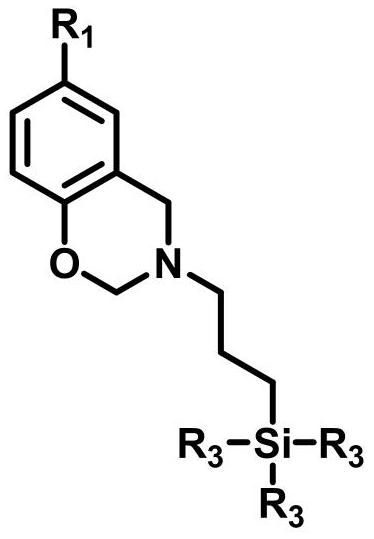
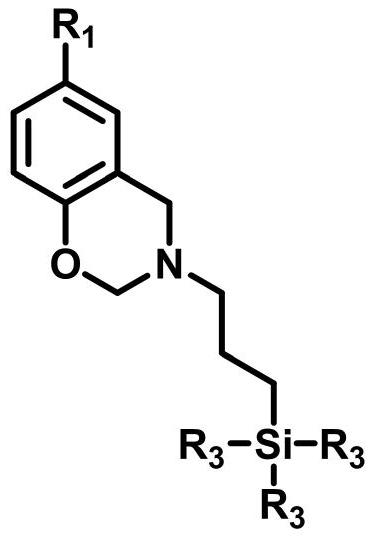
[0040] The first step, 3-trimethoxysiloxane-n-propyl-3,4-dihydro-6-methyl-2H-1,3-benzoxazine through literature (Langmuir.2011,27(13) :8365-8370) described method is synthesized, and synthetic steps are as follows:
[0041] In a 250mL four-neck flask equipped with mechanical stirring, condenser and thermometer 2After about 25-30min, add paraformaldehyde (0.1mol) and 50mL of chloroform, then add 7g of ground calcium hydride, and stir gently. When the temperature of the system rises to 65°C, when there is condensate dripping, add 3-aminopropyltrimethoxysilane (0.05mol), stir vigorously to raise the temperature, and when the temperature of the system rises to 85°C, add p-cresol (0.05mol), and the addition is complete Then reflux for 3h. After the reaction was completed, the residue was removed by filtration, and the solvent and other volatile impurities were distilled off under reduced pressure to obtain a viscous dark yellow liquid which was the target product benzoxazine-func...
Embodiment 2
[0056] The first step, the preparation method of 3-triethoxysiloxane-n-propyl-3,4-dihydro-6-dodecyl-2H-1,3-benzoxazine is the same as in Example 1, and the phenol used The compound p-cresol was replaced by 4-dodecylphenol to obtain the target product benzoxazine functionalized siloxane DP-eos. 1 H NMR (400MHz, deuterated chloroform, δ): 0.58-0.67,0.80-0.90,1.20-1.30,1.58-1.70,2.20-2.35,2.65-2.75,3.50-3.60,3.85-4.00,4.80-4.90,6.75- 7.15.
[0057] The 4-dodecylphenol, analytically pure, was purchased from Beijing Bailingwei Technology Co., Ltd. The 3-aminopropyltriethoxysilane, with a purity of ≥98%, was purchased from Hubei Debang Chemical New Material Co., Ltd.
[0058] The structure of the benzoxazine functionalized siloxane is as follows:
[0059]
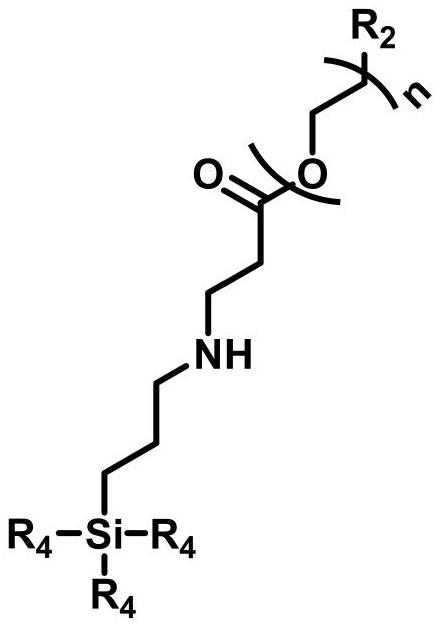
[0060] The preparation process of the polyethylene glycol functionalized siloxane CBPEG-mos with n being 200 is the same as that in Example 1. 1 H NMR (400MHz, deuterated chloroform, δ): 0.58-0.67, 1.30-1.50, 1.65-1.80, 2....
Embodiment 3
[0069] The first step, the preparation method of 3-triethoxysiloxane-n-propyl-3,4-dihydro-6-n-butyl-2H-1,3-benzoxazine is the same as in Example 1, for Cresol was replaced by 4-n-butylphenol to obtain the target product benzoxazine functionalized siloxane BP-eos. 1 HNMR (400MHz, deuterated chloroform, δ): 0.58-0.67,0.80-0.90,1.20-1.30,1.58-1.70,2.20-2.35,2.65-2.75,3.50-3.60,3.85-4.00,4.80-4.90,6.75-7.15 .
[0070] The 4-n-butylphenol, analytically pure, was purchased from Beijing Bailingwei Technology Co., Ltd. The 3-aminopropyltriethoxysilane, with a purity of ≥98%, was purchased from Hubei Debang Chemical New Material Co., Ltd.
[0071] The structure of the benzoxazine functionalized siloxane BP-eos is as follows:
[0072]
[0073] The preparation process of the polyethylene glycol functionalized siloxane SDPEG-eos with n being 20 is the same as that in Example 1. 1 H NMR (400MHz, deuterated chloroform, δ): 0.58-0.67, 1.15-1.25, 1.30-1.50, 1.65-1.80, 2.48-2.55, 2.90-3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com