Anti-IL-17RA monoclonal antibody and application thereof
A monoclonal antibody, expression vector technology, applied in the direction of antibodies, anti-receptors/cell surface antigens/cell surface determinants, immunoglobulins, anti-inflammatory agents, etc., to achieve the effect of preventing progression, alleviating symptoms, and strong affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 of the present invention provides an anti-IL-17RA monoclonal antibody, wherein the monoclonal antibody includes a heavy chain variable region and a light chain variable region, and the amino acid sequence of the heavy chain variable region is selected from one of the following sequences Species: SEQ ID NO:1, SEQ ID NO:2, SEQ ID NO:3, SEQ ID NO:4, SEQ ID NO:5, SEQ ID NO:6, SEQ ID NO:7, SEQ ID NO:8, SEQ ID NO:9 or SEQ ID NO:10; the amino acid sequence of the light chain variable region is selected from one of the following sequences: SEQ ID NO:11, SEQ ID NO:12 or SEQ ID NO:13.
[0045] The present invention utilizes a fully synthetic ScFv single-chain phage antibody library to screen and obtain a fully human monoclonal antibody specifically binding to IL-17RA. At present, fully human antibodies are the main direction for the development of therapeutic antibodies, and the emergence of antibody library technology provides a good technical platform for the preparat...
Embodiment 2
[0097] Embodiment 2 of the present invention also provides a polypeptide or protein comprising the anti-IL-17RA monoclonal antibody screened in Embodiment 1.
[0098] The present invention also provides a polynucleotide sequence or combination, the polynucleotide sequence or combination encodes the amino acid sequence of the anti-IL-17RA monoclonal antibody screened in Example 1.
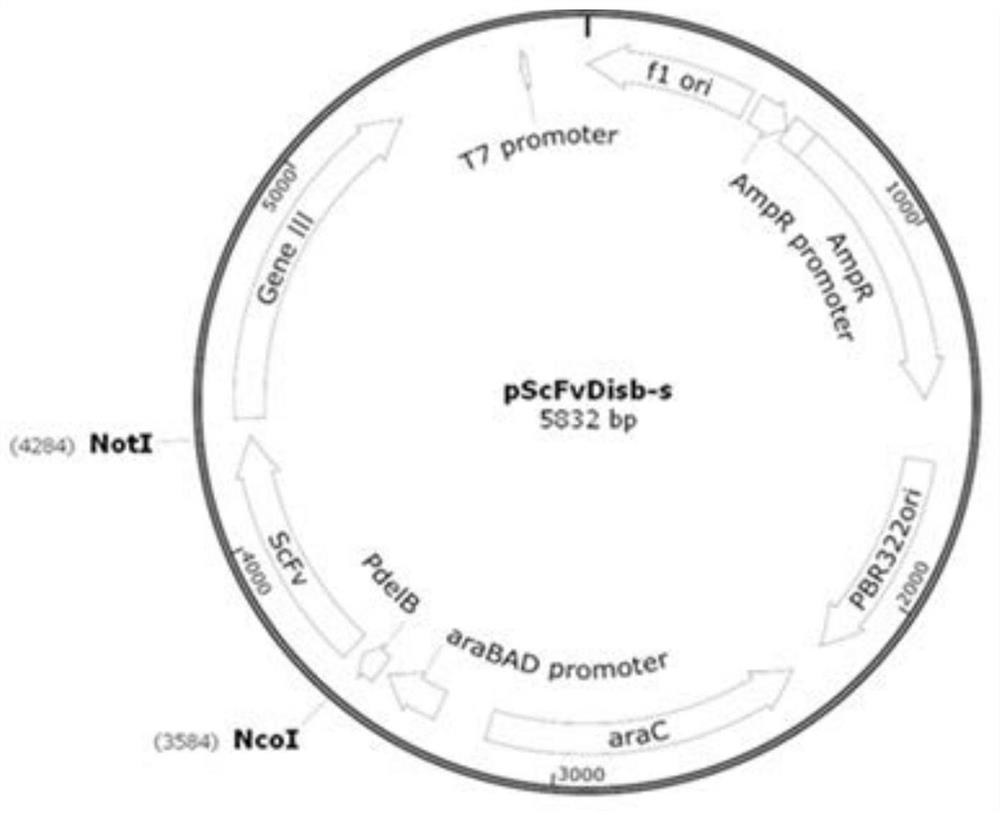
[0099] The present invention also provides a recombinant DNA expression vector, which comprises the above polynucleotide sequence or combination.
[0100] The present invention also provides a host cell transfected with the recombinant DNA expression vector, the host cell includes prokaryotic cells, yeast cells, insect cells or mammalian cells;
[0101] Preferably, the host cell is a mammalian cell, and the mammalian cell is HEK293E cell, CHO cell or NSO cell.
[0102] The present invention also provides a medicine or a pharmaceutical composition, which comprises the anti-IL-17RA monoclonal antibod...
Embodiment 3
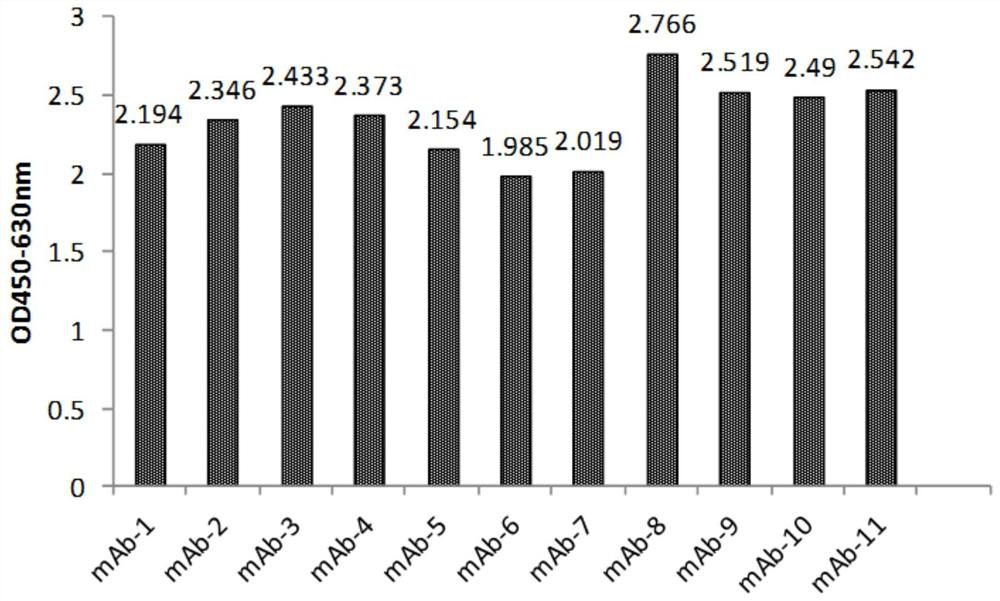
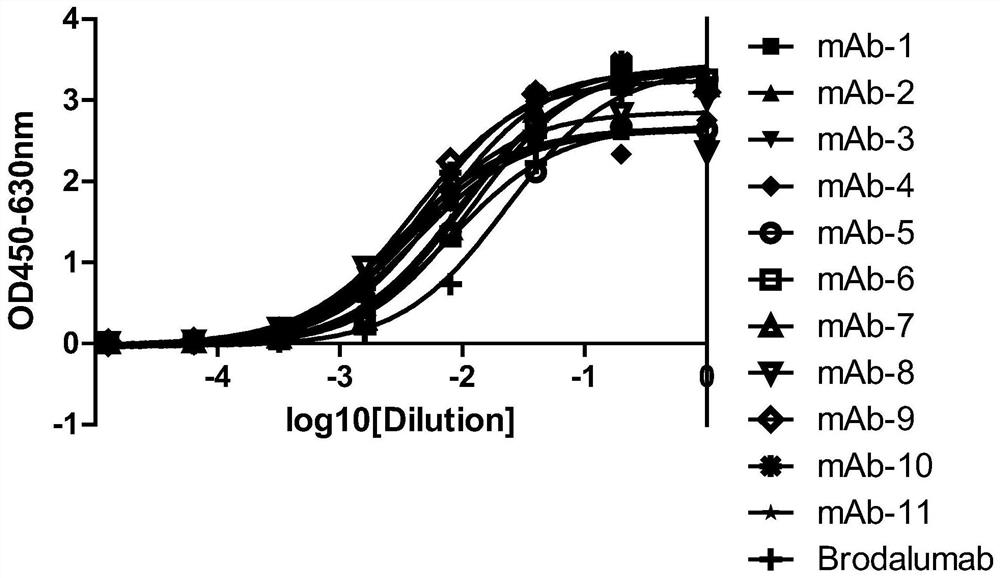
[0105] Example 3, Gradual dilution of phage Elisa to compare the affinity of anti-IL-17RA monoclonal antibodies
[0106] 3.1 Preparation of monoclonal antibody purified phage:
[0107] The 11 monoclonal antibodies (mAb-1, mAb-2, mAb-3, mAb-4, mAb-5, mAb-6, mAb-7, mAb-8, mAb-4, mAb-5, mAb-6, mAb-7, mAb-8, mAb -9, mAb-10, mAb-11) were transferred to 2YTAG liquid medium, shake cultured to the logarithmic growth phase, and then added M13K07 auxiliary phage infection, after centrifugation, the bacteria were resuspended in 2YTAKA, cultured at 28°C overnight and expanded The phages were amplified, and the phages were purified by sedimentation with PEG6000-NaCl the next day. The purified phage of the anti-IL-17RA monoclonal antibody (AMH14 / AML14) provided by the core patent US7833527B2 of the marketed product Brodalumab was used as a positive control.
[0108] 3.2 Affinity comparison at the phage level
[0109] IL-17RA-ECD-His was coated with 0.01M PBS buffer solution of pH 7.2, 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com