Preparation method of mesogen oxide and mesogen nitride, ammonia decomposition catalyst and preparation method
A catalyst and ammonia decomposition technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, catalyst activation/preparation, rare earth metal oxide/hydroxide, etc., can solve the problem of low catalytic activity and mesogenic oxide /Limited types of nitrides, environmental pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
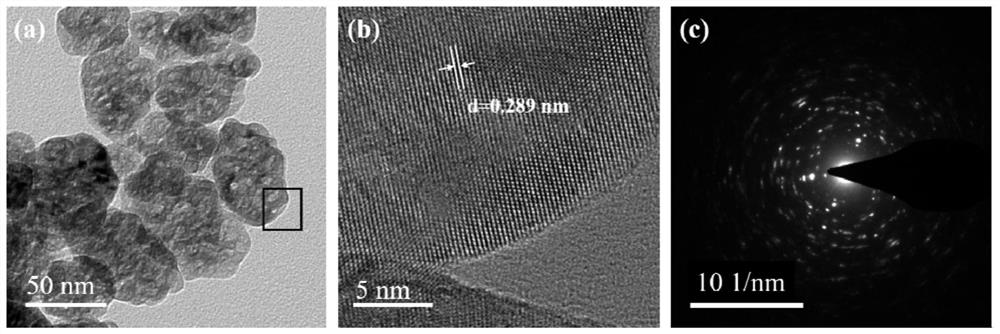
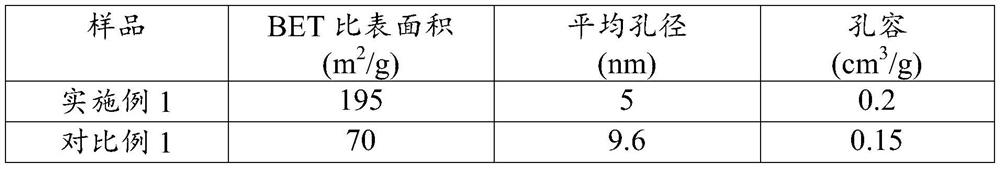
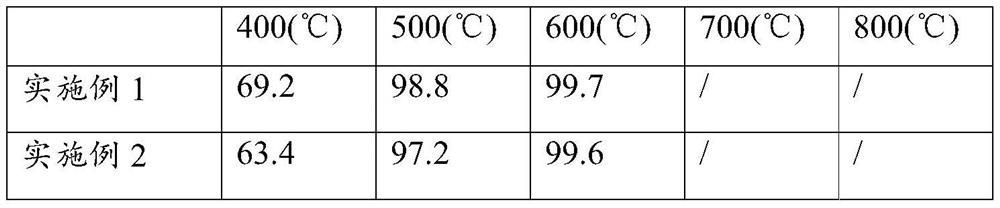
[0055] The present embodiment provides a kind of ammonia decomposition catalyst, with ZrO 2 The mesoscopic crystal is used as a carrier, and the active component is Ru, wherein, in terms of metal elements, the loading amount of the active component Ru is 3wt%.
[0056] The preparation method of this catalyst is as follows:
[0057] Weigh 11.2788g ZrOCl 2 ·8H 2 O was dissolved in 70ml deionized water and weighed 0.6475g NH 4 Add F to the above solution to obtain a mixed solution, and let it stand for 2 hours; transfer the mixed solution to a 100ml hydrothermal reaction kettle, and conduct a hydrothermal reaction at 200°C for 24 hours; after the hydrothermal kettle is naturally cooled to room temperature, centrifuge the hydrothermal product Wash until no Cl is detected - ; Then dry the obtained precipitate at 110°C for 12h, after drying, bake in a muffle furnace at 500°C for 8h to obtain ZrO 2 mesoscopic crystals.
[0058] The above 3.0g of ZrO 2 Add 45.9 mL of RuCl with ...
Embodiment 2
[0060] The present embodiment provides a kind of ammonia decomposition catalyst, with TiO 2 The mesoscopic crystal is used as a carrier, and the active component is Ru, wherein, in terms of metal elements, the loading amount of Ru is 3wt%.
[0061] The preparation method of this catalyst is as follows:
[0062] Weigh 8.9095g (NH 4 ) 2 TiF 6 Dissolve in 55mL of deionized water, then slowly add 15mL of 3mol / L ammonia solution dropwise; put the mixed solution in a constant temperature water bath at 25°C for 8h, then transfer it to a 100mL reactor for hydrothermal reaction at 130°C for 24h; Cool to room temperature, then centrifuge and wash with distilled water until Cl is no longer detectable - , dried at 110°C for 12h, and then calcined at 800°C for 2h to obtain TiO 2 mesoscopic crystals.
[0063] The above 3.0g of TiO 2 Add 45.9 mL of RuCl with a molar concentration of 0.02 mol / L to mesoscopic crystals 3 The ammonia decomposition catalyst was obtained by step-by-step im...
Embodiment 3
[0065] This embodiment provides an ammonia decomposition catalyst, which uses TiN mesoscopic crystals as a carrier, and the active component is Ru, wherein, calculated as metal elements, the loading amount of Ru is 3 wt%.
[0066] The preparation method of this catalyst is as follows:
[0067] Weigh 8.9095g (NH 4 ) 2 TiF 6 Dissolve in 55mL of deionized water, then slowly add 15mL of 3mol / L ammonia solution dropwise; put the mixed solution in a constant temperature water bath at 25°C for 8h, then transfer it to a 100mL reactor for hydrothermal reaction at 130°C for 24h; Cool to room temperature, then centrifuge and wash with distilled water until Cl is no longer detectable - , dried at 110°C for 12h, and then calcined at 800°C for 2h to obtain TiO 2 mesoscopic crystal; the prepared TiO 2 The mesoscopic crystals were heated slowly to 800°C in an ammonia atmosphere in a muffle furnace, and were nitrided for 2 hours to obtain TiN mesoscopic crystals.
[0068] Add 3.0 g of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com