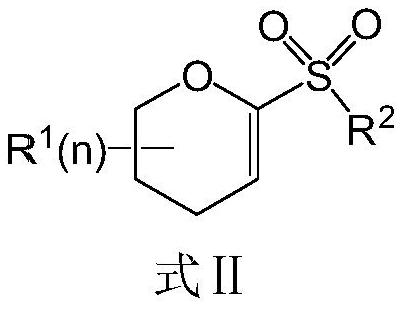
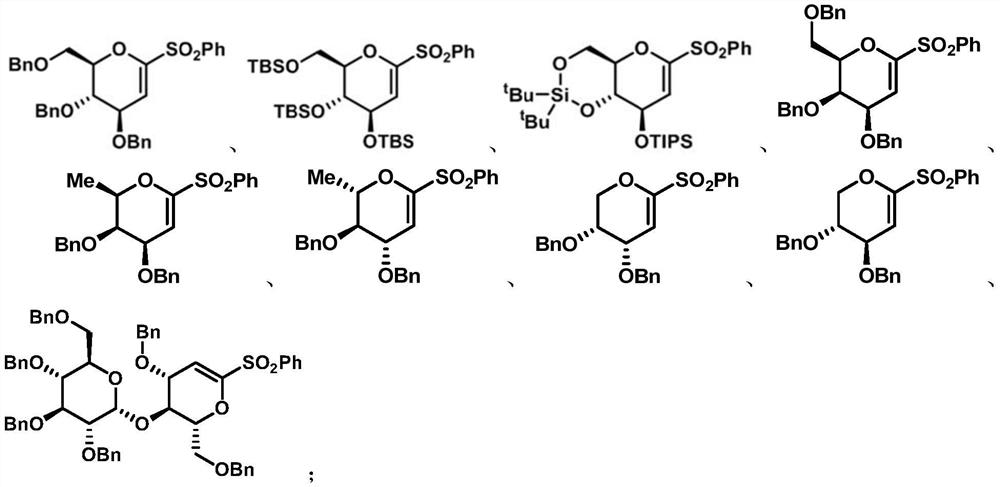
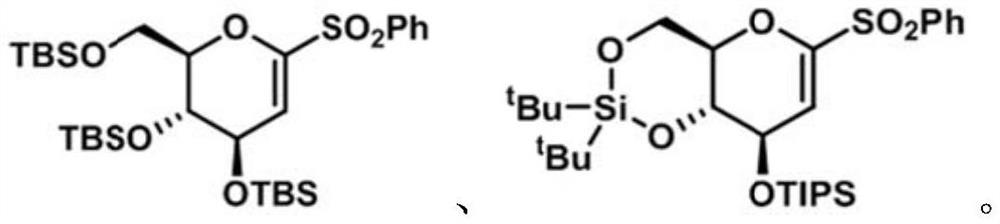
Preparation method of diabetes-treating medicine, namely ipragliflozin or derivative thereof
A technology of empagliflozin and derivatives, which is applied in the field of preparation of drug empagliflozin or derivatives thereof, and can solve the problems of restricting synthetic use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1, the preparation of electrophile α-O-alkenyl sulfone of the present invention
[0074] 1. Synthesis of 1-phenylsulfone-3,4,6-tribenzyloxy-D-glucene (8a)
[0075]
[0076] m-Chloroperbenzoic acid (mCPBA, 2.5g, 11.26mmol, 2.2equiv) was added in batches to dichloromethane ( DCM) solution, stirred at room temperature for 2 hours, and filtered with celite. Saturated NaHCO was added to the filtrate 3 and Na 2 S 2 o 3 The solution was stirred for 10 min, extracted with dichloromethane, and distilled under reduced pressure. Subsequent purification by chromatographic column (petroleum ether / ethyl acetate=4:1) gave SI-2 (2.65 g, 3.99 mmol) with a yield of 78%.
[0077] SI-2 (2.65g, 3.99mmol, 1.0equiv) in N 2 Dissolve in tetrahydrofuran (THF, 25mL) under protective conditions, stir at 0°C, then add lithium bistrimethylsilylamide (LiHMDS, 3.0mL, dissolved in n-hexane solution, concentration 2.0M, 1.5equiv) dropwise join in. Spot plate monitoring, use satura...
Embodiment 2
[0155] Embodiment 2, the influence of different process parameters on the Suzuki-Miyaura coupling reaction
[0156] 1. Effect of ligand on Suzuki-Miyaura coupling reaction
[0157] Taking the following reaction route as the basic reaction route, the influence of different ligands on the Suzuki-Miyaura coupling reaction was studied.
[0158]
[0159] Reaction result is as shown in table 1, and the structural formula of L1, L2, L3 and L4 in the table is as follows:
[0160]
[0161] Table 1 Effect of ligands on Suzuki-Miyaura coupling reaction
[0162]
[0163] a Reaction conditions (unless otherwise specified): 8a (0.05mmol, 1.0equiv), 9 (0.10mmol, 2.0equiv), THF (0.4mL). Yield and conversion were determined by 1 H NMR using 1,3,5-trimethoxybenzene as internal standard. b Ni(cod) 2 instead of Ni(OTf) 2 .
[0164] From the results in Table 1, it can be seen that the ligand is Cy 3 P·HBF 4 (i.e. Cy in Table 1 3 PHBF 4 ), the yield of the obtained compound is t...
Embodiment 3
[0198] Example 3. Preparation of aryl glycosides and open-chain alkenyl ethers using the α-O-alkenyl sulfone of the present invention as an electrophile in the Suzuki-Miyaura coupling reaction
[0199] The α-O-alkenyl sulfone (1 equivalent) prepared in Example 1, commercially available aryl boronic acid or borate (2 equivalents), Cy 3 P·HBF 4 (0.2 equivalent) was added to a screw-cap bottle with a magnetic stirrer, and the bottle cap was unscrewed before entering the glove box. Add Ni(COD) 2 (0.1 equiv), KOH (2 equiv), THF (0.2M, ie the concentration of α-O-alkenyl sulfone in THF is 0.2M). After tightening the small reaction bottle, remove it from the glove box, and seal the bottle mouth tightly with black tape. The mixed system was stirred at 60-80°C for 8-16 hours. The reaction solution was then cooled to room temperature, diluted with ethyl acetate (10 ml) and saturated with NH 4 After washing with Cl, it was extracted with ethyl acetate (10 mL x 3). Anhydrous Na for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com