Novel coronavirus pneumonia vaccine based on novel adenovirus vector sad23l and/or ad49l
A technology of ad49l-ncov-s and adenovirus, applied in virus/bacteriophage, microbe-based methods, viruses, etc., can solve problems such as limiting the application of adenovirus vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
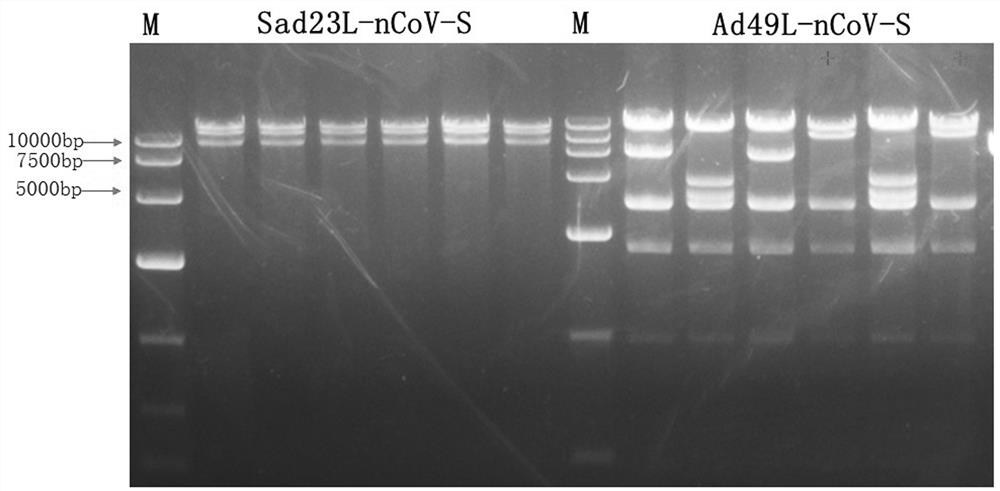
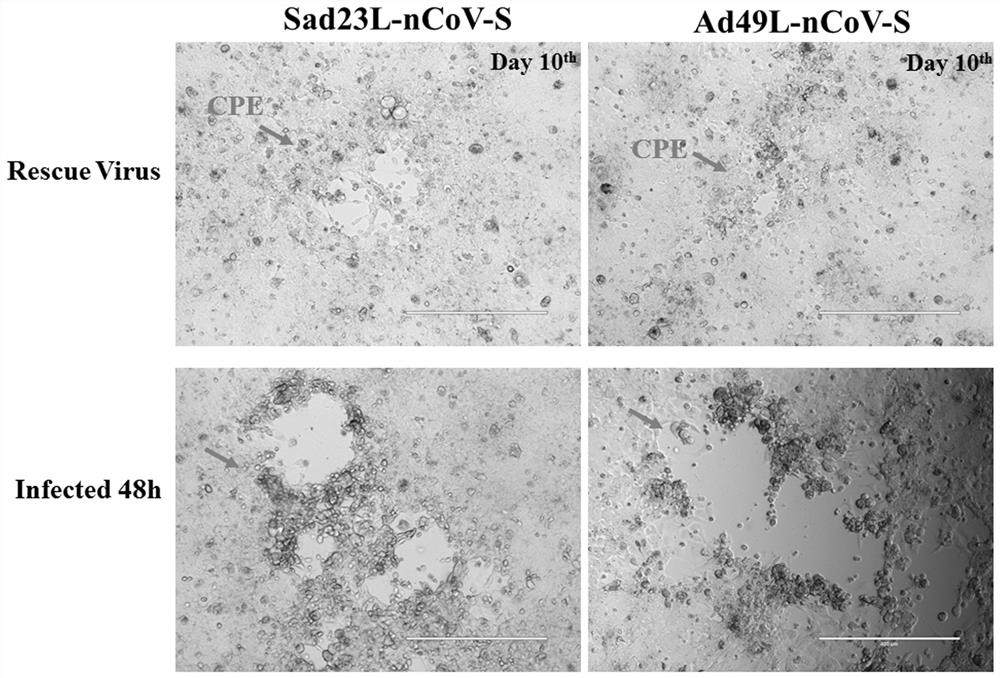
[0050] Example 1 Preparation of novel coronavirus vaccines based on replicated defective adenoviral carriers SAD23L and AD49L
[0051] 1. Codon optimization and acquisition of exogenous gene s.
[0052] SARS-COV-2 petrochemical protein (S) gene sequence derived from a new coronavirus strain (GenBank No. Mn908947.3), using software UpGene to optimize the optimization of the codon, so that the exogenous gene is more suitable for mammals The cells were expressed, and the gene sequence after the optimization of SEQ ID NO: 1 was found to obtain a plasmid PMV-NCOV-S obtained by obtaining an optimized exogenous gene sequence in China.
[0053] 2. Recombinant adenoviral vector construction and virus packaging.
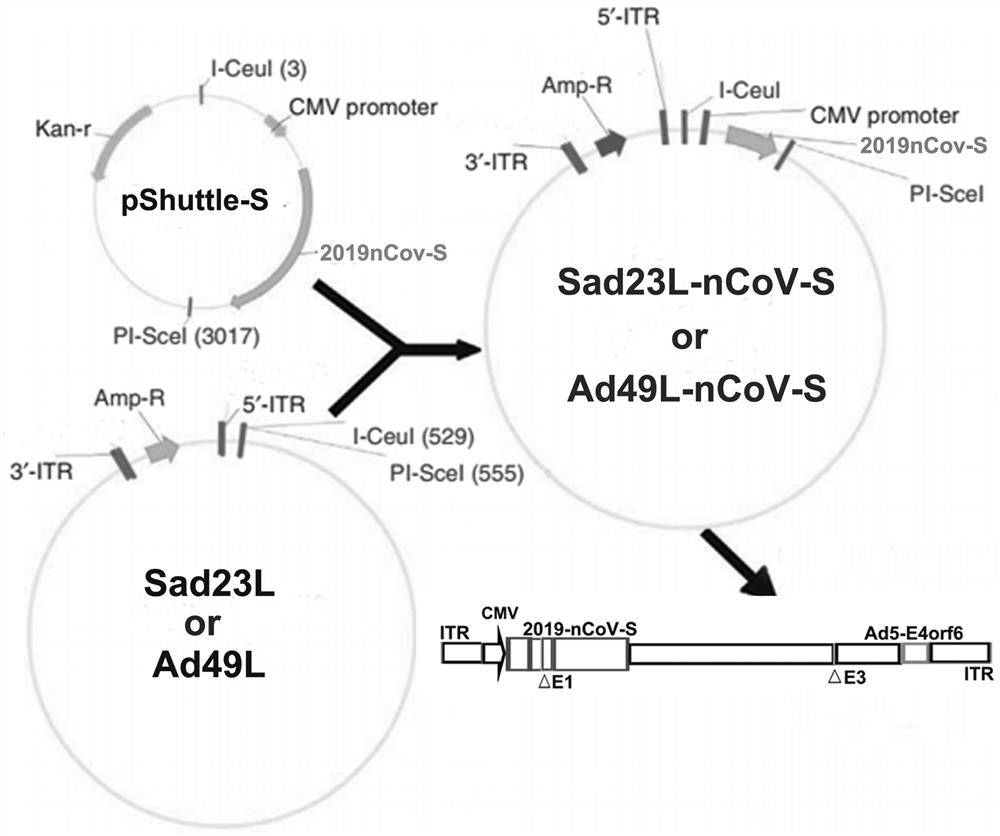
[0054] 2.1 Construction of shuttle plasmid pSHUTTLE2-CMV-S
[0055] Use the plasmid PMV-NCOV-S containing the gene sequence S of the whole gene EcoRi with Bamhi Differentiated, recovered enzyme digestion, and the product was ligated to the gluner PSHUTTLE2-CMV-FLAG for connection,...
Embodiment 2
[0073] Example 2 Immunization Evaluation of COVID-19 Vaccine SAD23L-NCOV-S in Mouse Model
[0074] 1. Recombinant adenovirus vaccine SAD23L-NCOV-S induced evaluation of specific body fluid immunity
[0075] 1.1 vaccination titer and site vaccination
[0076] The 5 weeks of SPF-class female C57BL / 6 mice were purchased from the South Medical University Animal Center, which was raised in the Southern Hospital Animal Center. All animal feeding and experiments are in line with national and institutional provisions on animal benefits. The injection volume is 100 μl, and the blood is taken from the blood, and the serum determination binding antibody and neutralizing antibody levels were isolated after immunization. The group of mice, as shown in Table 1:
[0077] Table 1: SAD23L-NCOV-S immunized mice packet, immunot dose and inoculation site
[0078]
[0079] 1.2 Vaccine immunization mice induced specificity for the binding antibody levels of s proteins and RBD proteins
[0080]After...
Embodiment 3
[0100] Example 3 Immunization Evaluation of COVID-19 Vaccine Pneumonia COVID-19 Vaccine AD49L-NCOV-S in Mouse Model
[0101] 1. Recombinant adenovirus vaccine AD49L-NCOV-S induced evaluation of specific body fluid immunity
[0102] 1.1 vaccination titer and site vaccination
[0103] The 5 weeks of SPF-class female C57BL / 6 mice were purchased from the South Medical University Animal Center, which was raised in the Southern Hospital Animal Center. All animal feeding and experiments are in line with national and institutional provisions on animal benefits. The injection volume is 100 μl, and the blood is taken from the blood, and the serum determination binding antibody and neutralizing antibody levels were isolated after immunization. The group of mice, the immunization is shown in Table 2:
[0104] Table 2: AD49L-NCOV-S immunized mice group, immunot dose and inoculation site
[0105]
[0106] 1.2 Vaccine immunization mice induced specificity for the binding antibody levels of s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com