A kind of preparation method of coronene compound
A technology of compounds and benzenes, applied in the field of organic chemistry, can solve problems such as complicated operations, unsuitable for large-scale production, and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Synthesis of DNL-6 molecular sieve catalyst
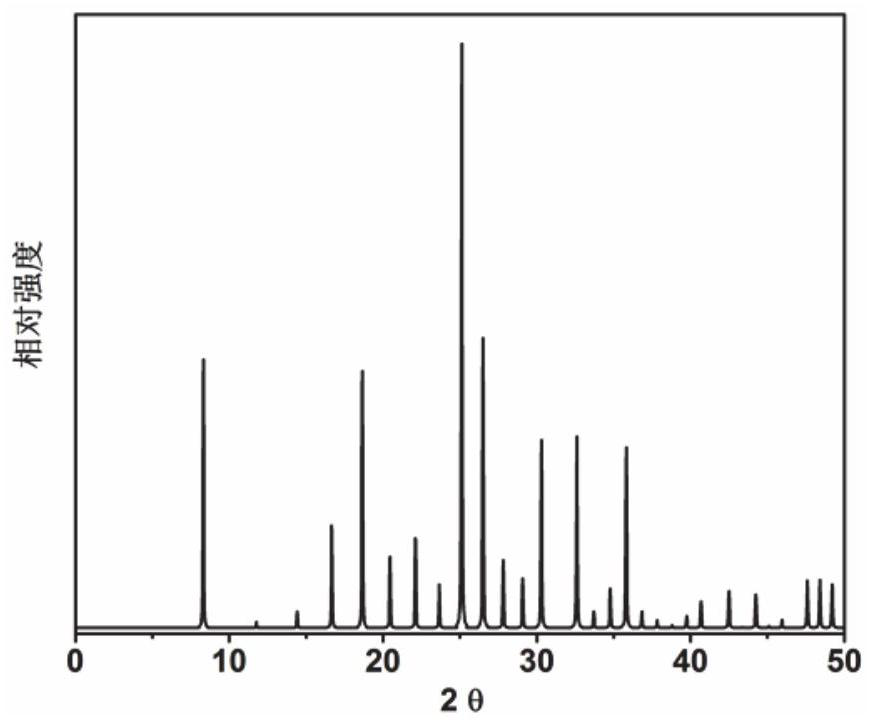
[0054] Initial gel Moore composition ratio 2N-methylbutylmine (as an organic structural guide): 0.3 Sio 2 : 0.4P 2 O 5 : 0.5al 2 O 3 : 50h: 50h 2 O: 0.2 (20%) of hexadalkyltrimel bromide (as seed) will meter the organic structural guide, silica sol, phosphoric acid, isopropanol, deionized water, and seed crystals in a beaker The gel is fully stirred, and then loaded into the stainless steel autoclave of the inner lining polytetrafluoroethylene, at a constant temperature of 200 ° C, a rotational crystal crystallization of 75 R / min. After crystallization, the centrifuge 3000 rp / separated center was 3 min, and the obtained solid product was washed with deionized water, and after drying overnight at 120 ° C, XRD analysis results figure 1 Indicated. As can be seen from the results of Fig. 1, the synthetic product is a DNL-6 molecular sieve powder, which is calcined at 550 ° C for 5 h to obtain a DNL-6 molecular sieve, ...
Embodiment 2
[0055] Example 2: Synthesis of SAPO-42 molecular sieve catalyst
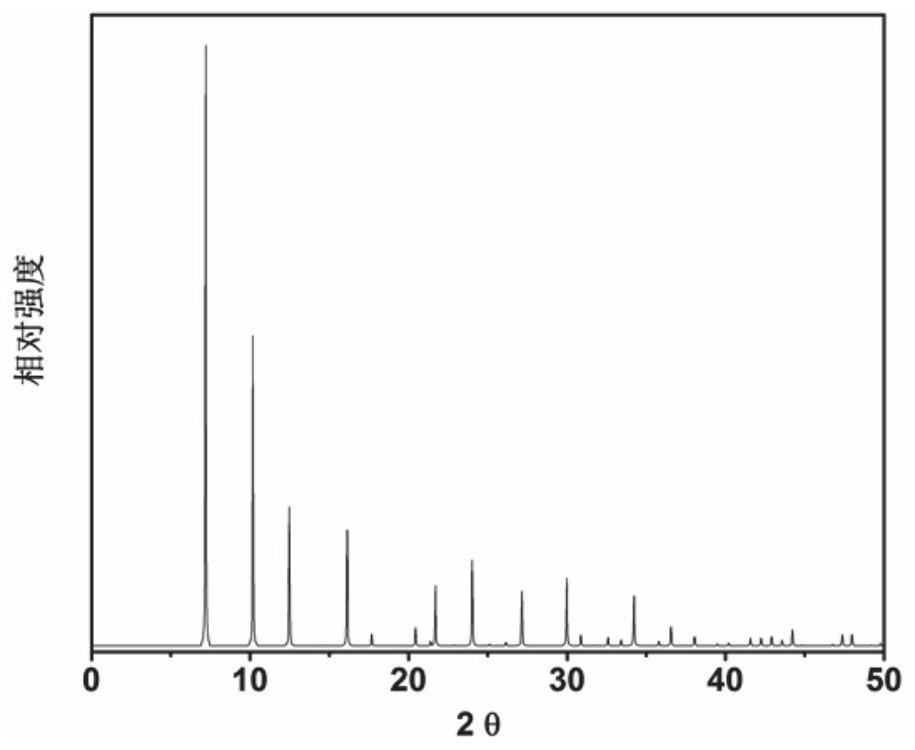
[0056] Proportion of 1.85 2,2-dimethyl-2,3-dihydro-1H-phenyl group [DE] isoquinoline (as an organic structural guide): 0.296sio 2 : 0.85P 2 O 5 : 1Al 2 O 3 : 60h 2 O The metered organic structural guides, silica sol, phosphoric acid, and thin aluminum stone and deionized water are mixed in the beaker, stir well, then loaded into the stainless steel autoclave of the inner lining polytetrafluoroethylene, 175 ° C constant temperature crystallization 120h. After crystallization, 3 min 300 rutthest centrifuge was crystallized, and the obtained solid product was washed with deionized water, and the XRD analysis results after drying overnight in air at 100 ° C. figure 2 Indicated. From figure 2 As a result, it can be seen that the synthetic product is SAPO-42 molecular sieve powder powder, which is calcined at 600 ° C for 5 h to obtain SAPO-42 molecular sieve, which is recorded as a catalyst 2.
Embodiment 3
[0057] Example 3: Synthesis of ZK-21 molecular sieve catalyst
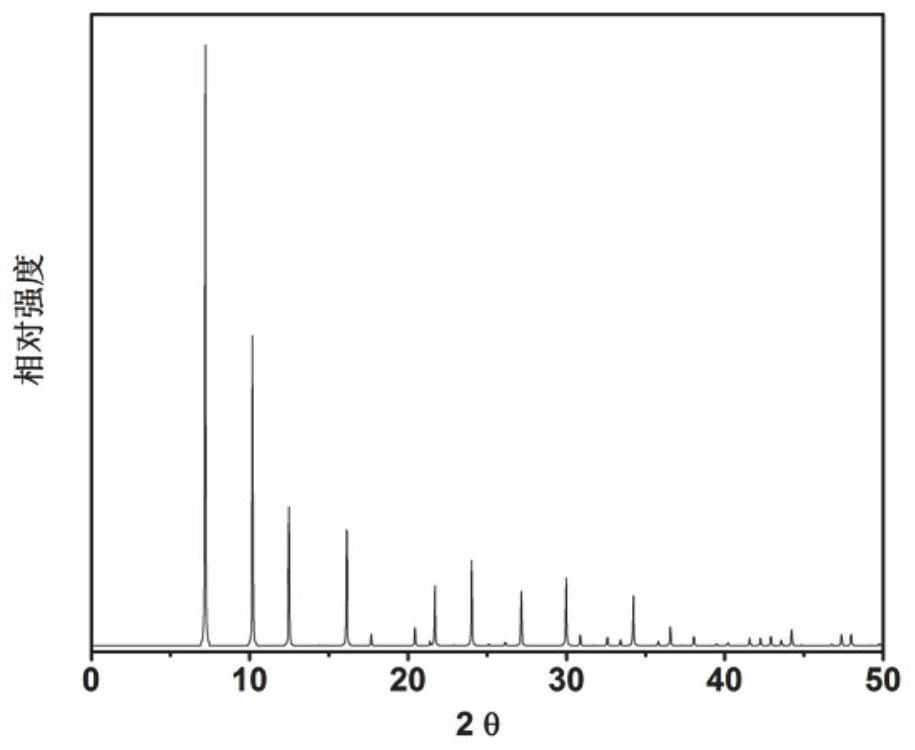
[0058] 6.1 g of aluminate (1.11NA) 2 O · Al 2 O 3 · 3.5H 2 O) mixed with a small amount of deionized water, adding 23 g of concentration of 85% phosphoric acid, an aqueous solution of aqueous solution, and 10.3 g of sodium biased silicate five hydrate and 80 ml of deionized water, and thoroughly agitated, then gave obtained The stainless steel autoclave loaded into the lined polytetrafluoroethylene was 100 ° C. After crystallization, 3 min 300 rutthest centrifuge was crystallized, and the obtained solid product was washed with deionized water, and the XRD analysis results after drying overnight in air at 100 ° C. image 3 Indicated. From image 3 As a result, it can be seen that the synthetic product is a ZK-21 molecular sieve powder, which is calcined at 600 ° C for 5 h, and it is obtained to obtain a ZK-21 molecular sieve, which is recorded as a catalyst 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com