A biocompatible antimicrobial peptide with self-assembly potential, its preparation method and application
A biocompatible, antimicrobial peptide technology, applied to biocompatible antimicrobial peptides with self-assembly potential and their preparation and application fields, can solve the problems of low cytotoxicity of antibacterial activity and poor cell selectivity of natural antimicrobial peptides with strong toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Design and preparation of embodiment 1 antimicrobial peptide synthesis
[0013] Combining diphenylalanine dipeptide FF with Ile to introduce hairpin structure D The PG corner unit stabilizes the internal hydrophobic structure and makes it a hydrophobic assembly motif. Combined with the amino acid composition required for the design of antibacterial peptides, the positively charged amino acid arginine and the hydrophobic amino acid leucine are selected as repeating binary sequence units and distributed on both sides. On the other hand, to ensure the hydrophobicity and positive charge required for its antibacterial function, the antibacterial peptide F2I-LL was newly designed, and its amino acid sequence is shown in Table 1.
[0014] The amino acid sequence of table 1 antimicrobial peptide FI-LL
[0015]
[0016] The charge number of F2I-LL is +5, the hydrophobic value is 0.716, and the hydrophobic moment is 0.198. N-terminal acetylation increases stability by adding...
Embodiment 2
[0018] 1 Bacteriostatic activity of antimicrobial peptides
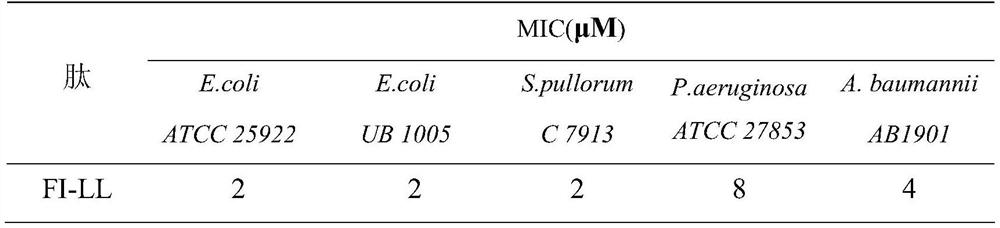
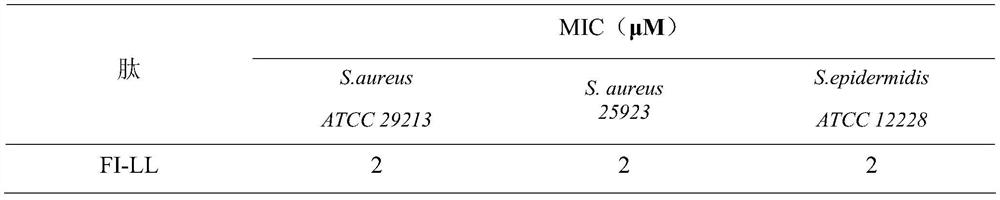
[0019] The peptide was configured as a 2.56mM / L storage solution for future use. The minimum inhibitory concentrations of several antimicrobial peptides were determined by the broth microdilution method. Using 0.01% acetic acid (containing 0.2% BSA) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 100 μL of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 individual / mL) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Incubate at a constant temperature of 37°C for 18 hours, and the minimum inhibitory concentration is the one where no turbidity is seen at the bottom of the well with the naked eye. The results are shown in Table 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com