Multi-bioactivity modified lignin self-assembled drug-loaded nano-micelle and preparation method thereof
A biologically active, drug-loaded nanotechnology, which is applied in the direction of pharmaceutical formulations, organic active ingredients, and medical preparations of non-effective ingredients, can solve problems that have not been related to research, and achieve abundant raw material sources, low cost, and simple processes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
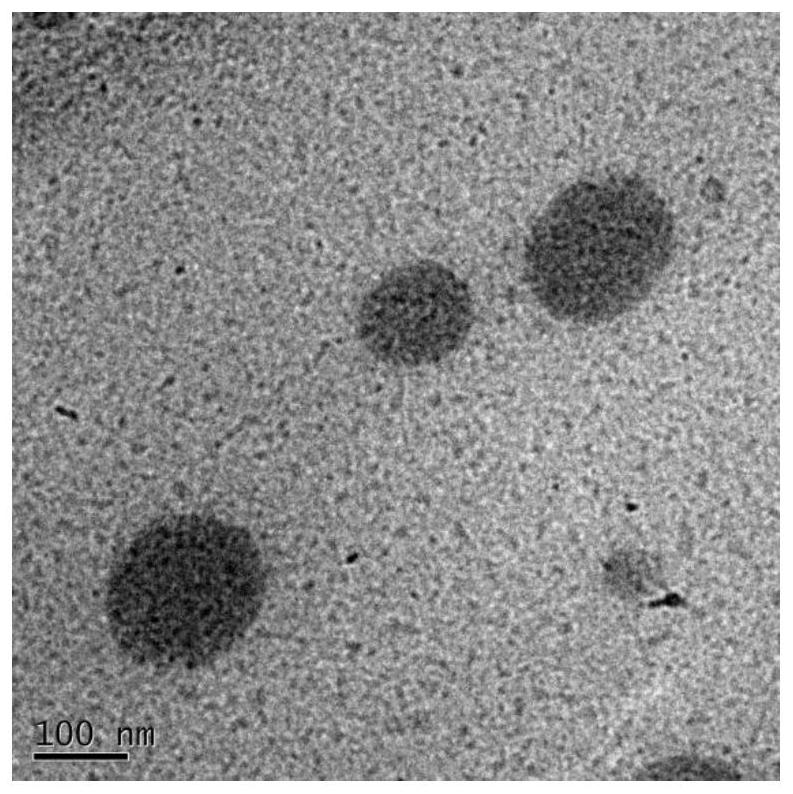
Image
Examples
Embodiment 1
[0036] A method for preparing multi-biologically active modified lignin self-assembled drug-loaded nano-micelles, specifically comprising the following steps:
[0037] Step 1, the lignin of 2.00g, 0.49g (5.0mmol-CO 2 ) of maleic anhydride, 25mg (0.2mmol) of 4-dimethylaminopyridine (DMAP) were added to 30mL of N,N-dimethylformamide (DMF), and reacted in a constant temperature water bath at 50°C for 4h while stirring. After the reaction, add 300mL of pure water to make it precipitate, filter, wash with pure water three times, then wash with absolute ethanol three times, and dry under vacuum at 50°C to obtain acylated lignin, that is, maleylated lignin (MAL);
[0038]Step 2. Dissolve 2.0 g of MAL, 0.15 g (0.50 mmol) of ellagic acid (EA) and 3 mg (0.025 mmol) of 4-dimethylaminopyridine (DMAP) in 10 mL of DMF, and add to a 100 mL round bottom flask After stirring for 5 min, slowly add 10 mL of 114 mg (0.6 mmol) of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride dropwis...
Embodiment 2-8
[0046] The parameter comparison table of embodiment 1 and embodiment 2-8 is shown in table 1 for details:
[0047] Among them: alkali lignin, enzymatic lignin, kraft lignin and kraft lignin are represented by A1-A4 respectively; maleic anhydride, phthalic anhydride and succinic anhydride are represented by B1-B3 respectively; ellagic acid, white Veratrol and curcumin are represented by C1-C3 respectively.
[0048] The parameter comparison table of table 1 embodiment 1-8
[0049]
[0050] The process steps and other process parameters of above-mentioned embodiment 2-8 are the same as embodiment 1.
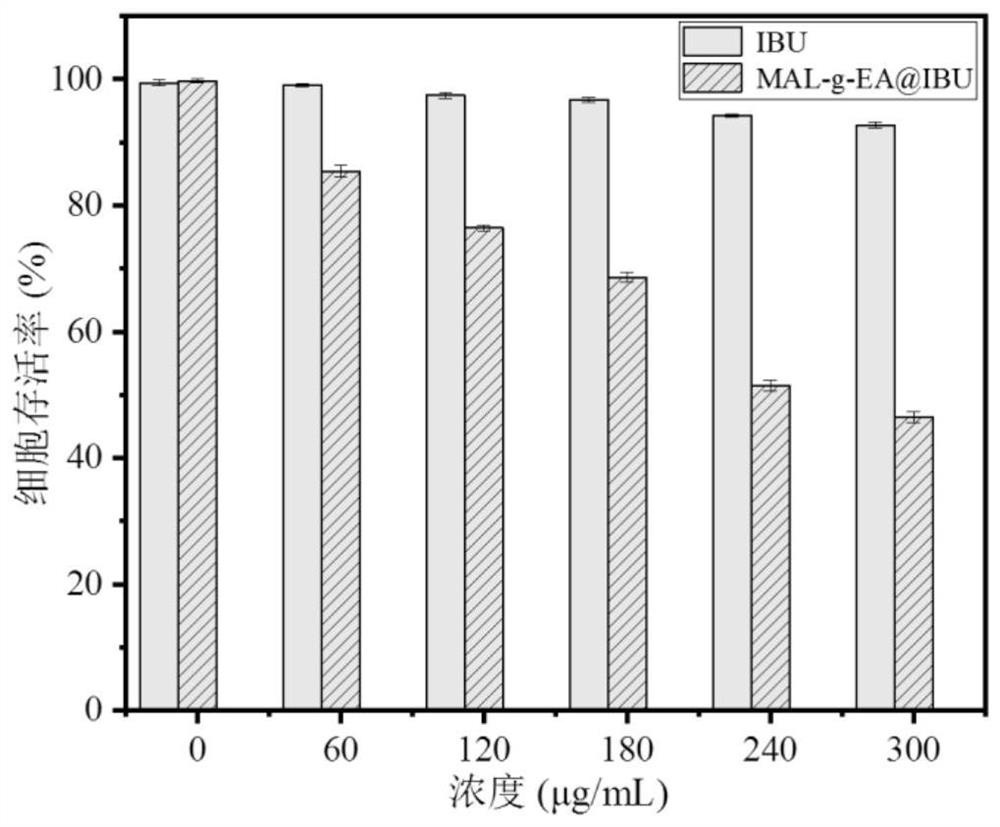
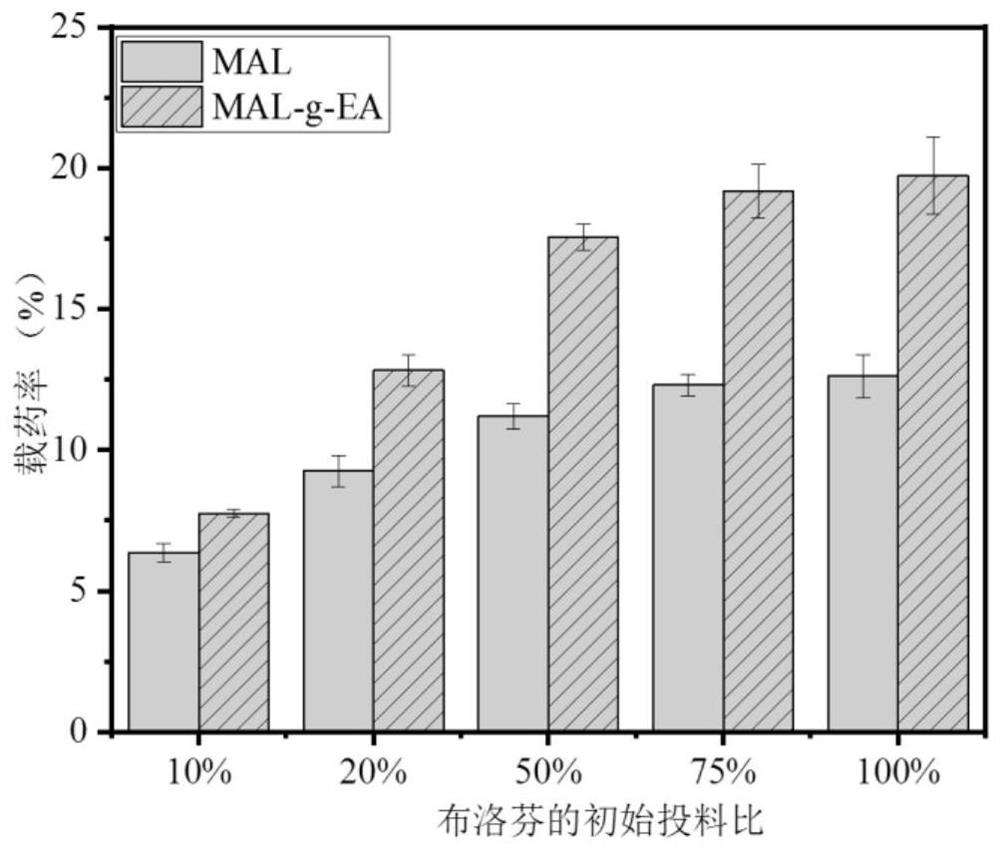
[0051] See Table 2 for the drug loading data of Examples 1-8.
[0052] Table 2 The drug loading rate table of embodiment 1-8 product entrapped ibuprofen (the initial charging amount of IBU is 50%)
[0053] serial number Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Example 7 Example 8 Drug loading rate (%) 17.55 15.637 15.79 15....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com