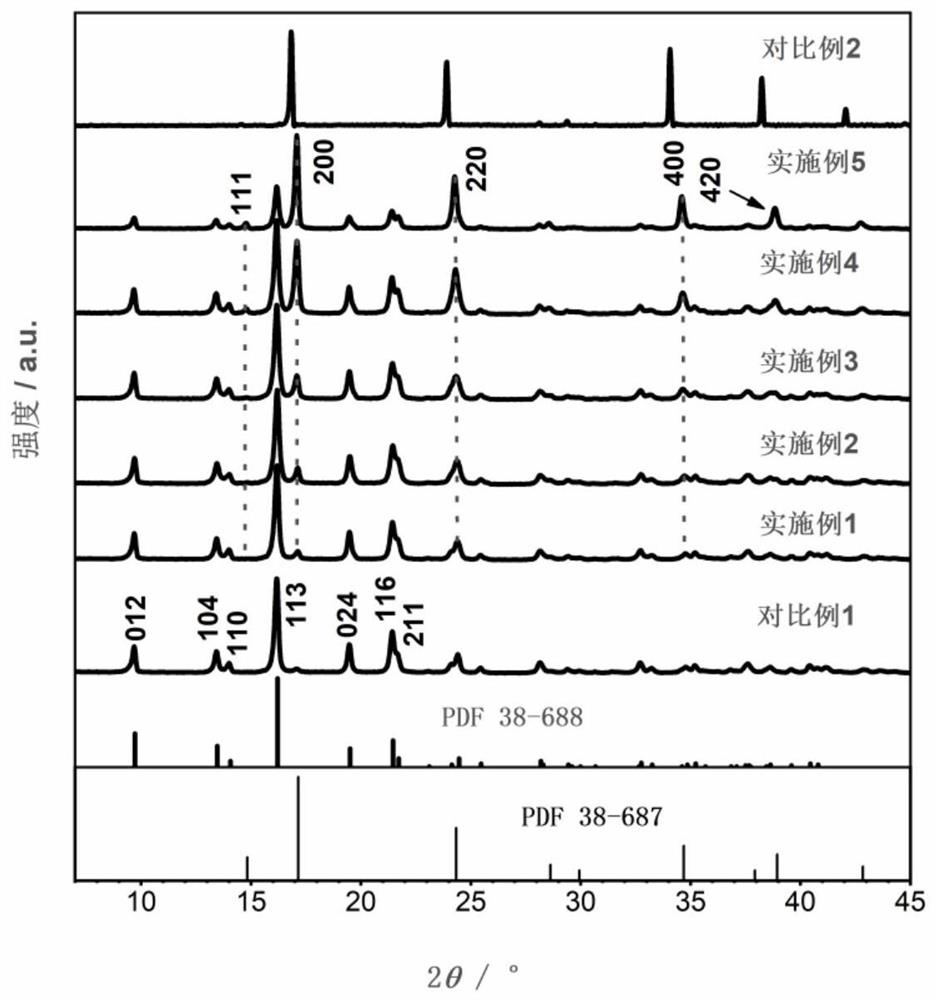
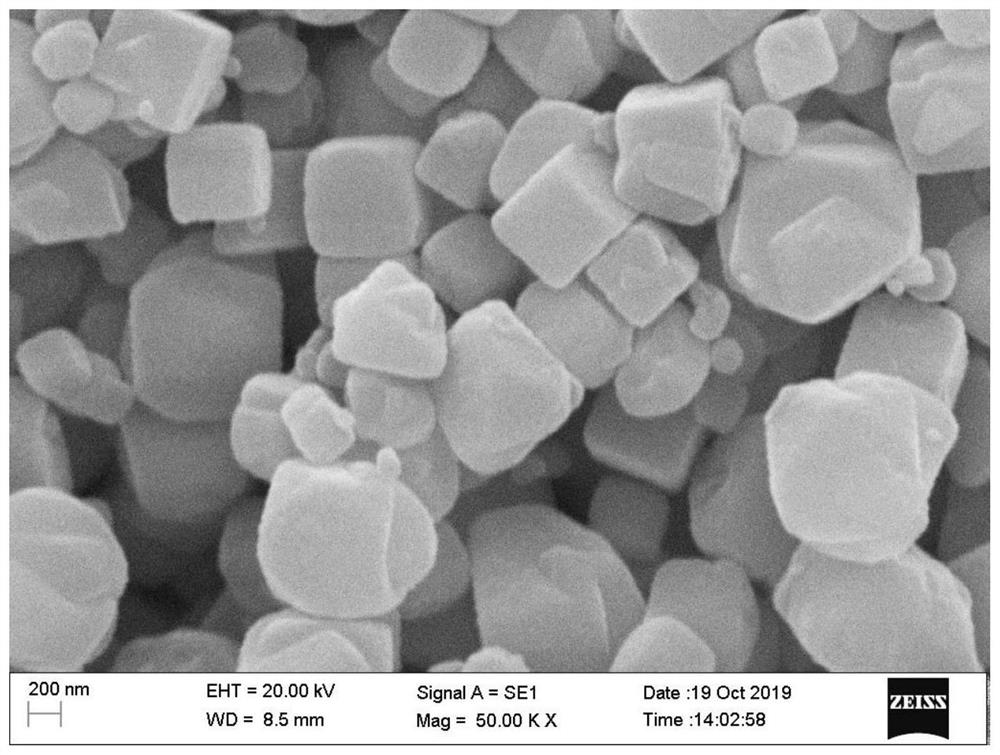
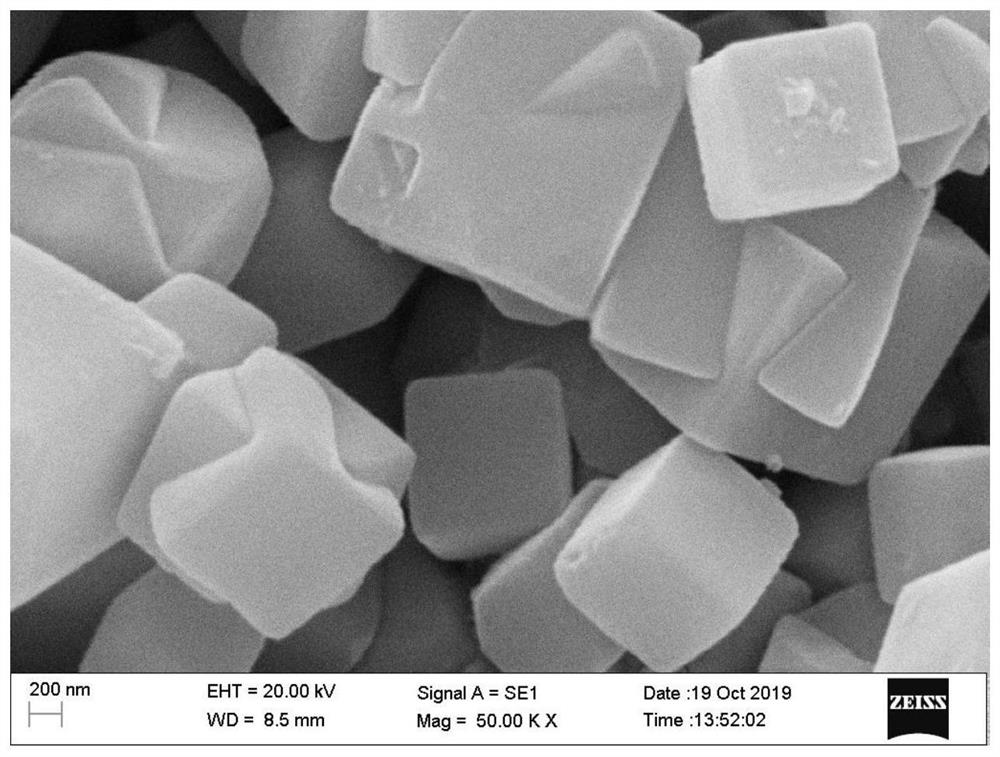
Manganese-doped modified zinc ion battery positive electrode active material and preparation method and application thereof
A positive electrode active material, zinc-ion battery technology, applied in the direction of battery electrodes, positive electrodes, secondary batteries, etc., can solve the problems of difficult rapid migration of zinc ions, capacity decline, short cycle life, etc., to improve cycle stability, improve The effect of cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The present invention also provides a method for preparing the above positive electrode material, which is characterized in that it comprises the following steps:
[0062] After mixing the raw materials comprising the positive electrode active material, the conductive agent and the binder, a dispersant is added, and the positive electrode material is formed after mixing.
[0063] Preferably, in the above preparation method, the dispersant is selected from water, ethanol, N-methylpyrrolidone, isopropanol or methanol.
[0064] After mixing the raw materials comprising the positive electrode active material, the conductive agent and the binder, a dispersant is added, the resulting product is coated on the conductive current collector, and the zinc ion battery electrode is obtained after drying.
[0065] Preferably, the conductive current collector is selected from carbon paper, carbon cloth, carbon felt, titanium foil, stainless steel foil, copper foil, aluminum foil, nick...
Embodiment 1
[0103] Preparation of Mn 0.03 Zn 2.97 [Fe(CN) 6 ] 2 ·yH 2 O (referred to as MZHCF), the steps are as follows:
[0104] (1) Weigh 0.5693g ZnSO 4 ·7H 2 O and 0.0035g Mn (NO 3 ) 2 Add 100mL of deionized water to prepare 0.0198mol / L ZnSO 4 and 0.0002mol / L Mn(NO 3 ) 2 mixture;
[0105] (2) Weigh 0.6585g K 3 Fe(CN) 6 , add 100 mL of deionized water to make 0.02 mol / L K 3 Fe(CN) 6 aqueous solution;
[0106] (3) 100mL ZnSO 4 with Mn (NO 3 ) 2 The mixed solution was added to 100mL K at a rate of 50mL / h 3 Fe(CN) 6 In the aqueous solution, and maintain vigorous stirring (stirring speed is 500rpm), form a mixture;
[0107] (4) the mixture kept vigorous stirring (stirring speed was 500rpm) for 12 hours, and then left standstill for 6 hours to obtain the product;
[0108] (5) Wash the reaction product with deionized water and ethanol, and dry it at 70° C. for 12 hours to obtain Mn 0.03 Zn 2.97 [Fe(CN) 6 ] 2 ·yH 2 O solid.
Embodiment 2
[0110] Preparation of Mn 0.09 Zn 2.91 [Fe(CN) 6 ] 2 ·yH 2 O, the steps are as follows:
[0111] (1) Weigh 0.5579g ZnSO 4 ·7H 2 O and 0.0107g Mn (NO 3 ) 2 Add 100 mL of deionized water to prepare 0.0194 mol / L ZnSO 4 and 0.0006mol / L Mn(NO 3 ) 2 mixture;
[0112] (2) Weigh 0.6585g K 3 Fe(CN) 6 , add 100 mL of deionized water to make 0.02 mol / L K 3 Fe(CN) 6 aqueous solution;
[0113] (3) 100mL ZnSO 4 with Mn (NO 3 ) 2 The mixed solution was added to 100 mL K at a rate of 100 mL / h. 3 Fe(CN) 6 In the aqueous solution, and maintain vigorous stirring (stirring speed is 800rpm), form a mixture;
[0114] (4) the mixture kept vigorous stirring (stirring speed was 800rpm) for 12 hours, and then left standstill for 6 hours to obtain the product;
[0115] (5) Wash the reaction product with deionized water and ethanol, and dry it at 70° C. for 12 hours to obtain Mn 0.09 Zn 2.91 [Fe(CN) 6 ] 2 ·yH 2 O solid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com