Sensing array with mitochondrial targeting and aggregation-induced emission effects and application of sensing array in cell discrimination
A technology of aggregation-induced luminescence and sensing array, which is applied in the application field of sensing array with mitochondrial targeting and aggregation-induced luminescence effect and cell discrimination, and can solve the problems of low accuracy, low sensitivity of cancer, complicated operation steps, etc. , to achieve the effect of easy synthesis, good biocompatibility and photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
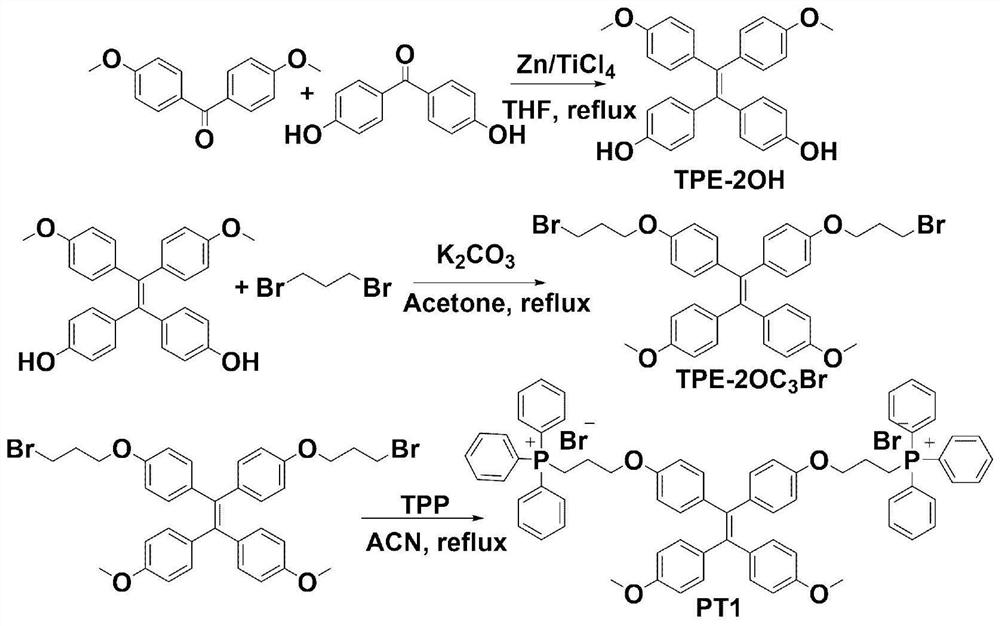
[0041] Embodiment 1: Synthesis of PT1
[0042] Structural formula: Synthesis process see figure 1 The synthetic route shown.
[0043] (1) Synthesis of TPE-2OH: get 4,4'-dihydroxybenzophenone (3.00g, 14mmol), 4,4'-dimethoxybenzophenone (3.39g, 14mmol) and zinc powder ( 7.28g, 112mmol) into a 250mL two-necked flask, add a magnet, add anhydrous THF 150mL, deoxygenate and keep N 2 environment. In an acetone bath, slowly to N 2 Titanium tetrachloride (6.15 mL, 56 mmol) was added dropwise into a protected two-necked flask, stirred in an acetone bath for 0.5 h, returned to room temperature naturally, and then refluxed for 9 h. After the reaction was completed, room temperature was restored, and saturated potassium carbonate solution was added to the mixture to quench the reaction. The zinc powder was removed by suction filtration, and the filtrate was extracted with water and dichloromethane (100 mL*3). The organic phase was collected, washed with water, and dried over anhydr...
Embodiment 2
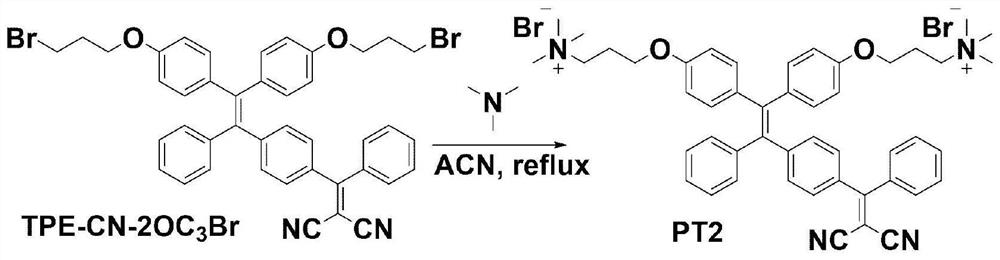
[0046] Embodiment 2: Synthesis of PT2
[0047] Structural formula: Synthesis process see figure 2 The synthetic route shown.
[0048] Synthesis of PT2: Take TPE-CN-2OC 3 Br (100.00mg, 0.13mmol) and Me 3 N (76.83mg, 1.3mmol) was added to a 50mL one-necked flask, and 30mL of anhydrous THF was added to remove oxygen, and stirred at 25°C for 2 days. After the reaction was completed, the mixture was filtered and the residue was washed with excess THF to obtain a red solid (52.61 mg, 0.06 mmol, yield: 45%). 1 H NMR (400MHz, CD 3 OD,δ):7.71(d,J=7.2Hz,1H),7.64–7.55(m,1H),7.54–7.49(m,2H),7.48–7.41(m,1H),7.40–7.28(m, 1H),7.26–7.09(m,4H),7.08–6.98(m,3H),6.98–6.82(m,5H),6.79–6.65(m,4H),4.04(t,J=4.8Hz,4H) ,3.56(t,J=8.0Hz,4H),3.17(s,18H),2.31–2.17(m,4H); HR-MS(ESI,positive)m / z:[M-2Br] 2+ / 2calcd.for[C 48 h 52 N 4 o 2 ] 2+ / 2,358.20396; found, 358.20413.
Embodiment 3
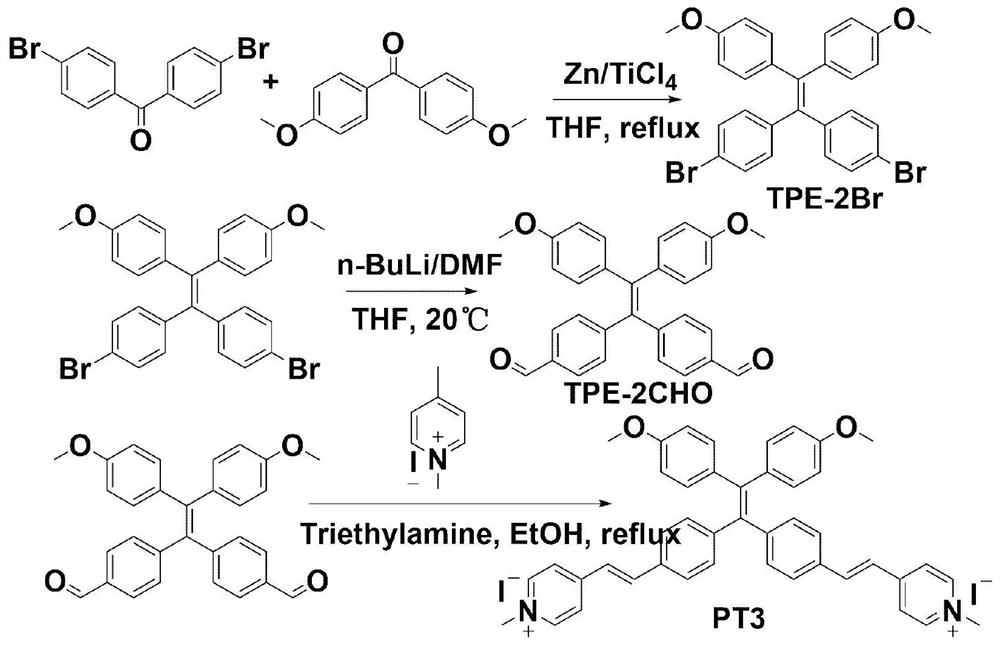
[0049] Embodiment 3: synthetic PT3
[0050] Structural formula:
[0051] Synthesis process see image 3 The synthetic route shown.
[0052] (1) Synthesis of TPE-2Br: Take 4,4'-dibromobenzophenone (3.00g, 8.82mmol), 4,4'-dimethoxybenzophenone (2.13g, 8.82mmol) and zinc Powder (7.28g, 70.56mmol) was added to a 250mL two-necked flask, a magnet was added, anhydrous THF 150mL was added, and oxygen was removed to keep N 2 environment. In an acetone bath, slowly to N 2 Titanium tetrachloride (6.15 mL, 35.82 mmol) was added dropwise into a protected two-neck flask, stirred in an acetone bath for 0.5 h, returned to room temperature naturally, and then refluxed for 9 h. After the reaction was completed, room temperature was restored, and saturated potassium carbonate solution was added to the mixture to quench the reaction. The zinc powder was removed by suction filtration, and the filtrate was extracted with water and dichloromethane (100 mL*3). The organic phase was collected...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com