Crystal form of amantadine compound, and preparation method thereof
A technology for compounds and crystal forms, applied in the field of crystal forms of amantadine compounds and their preparation, can solve problems such as difficulty in research and production, use process, inability to detect and determine microscopic morphology, unfavorable research on pharmaceutical preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Example 1: Preparation of ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methyl palmitate crystal form A
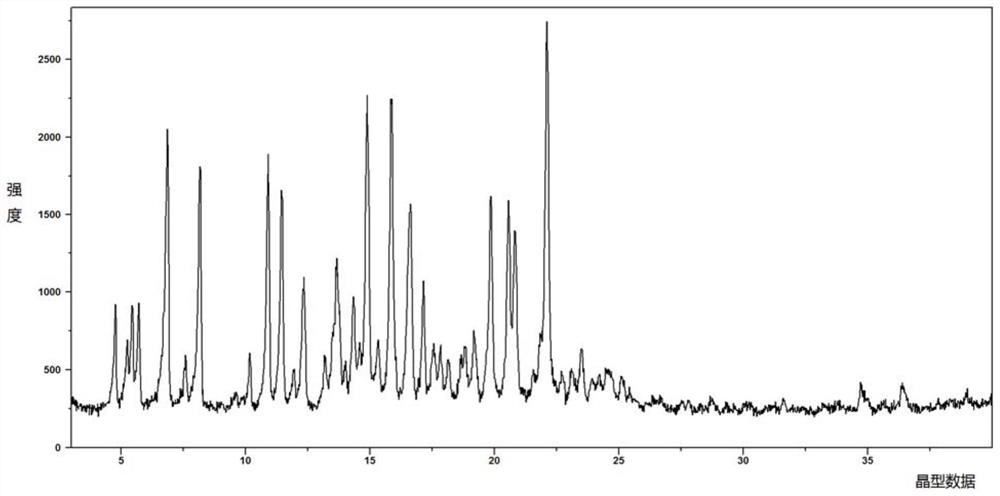
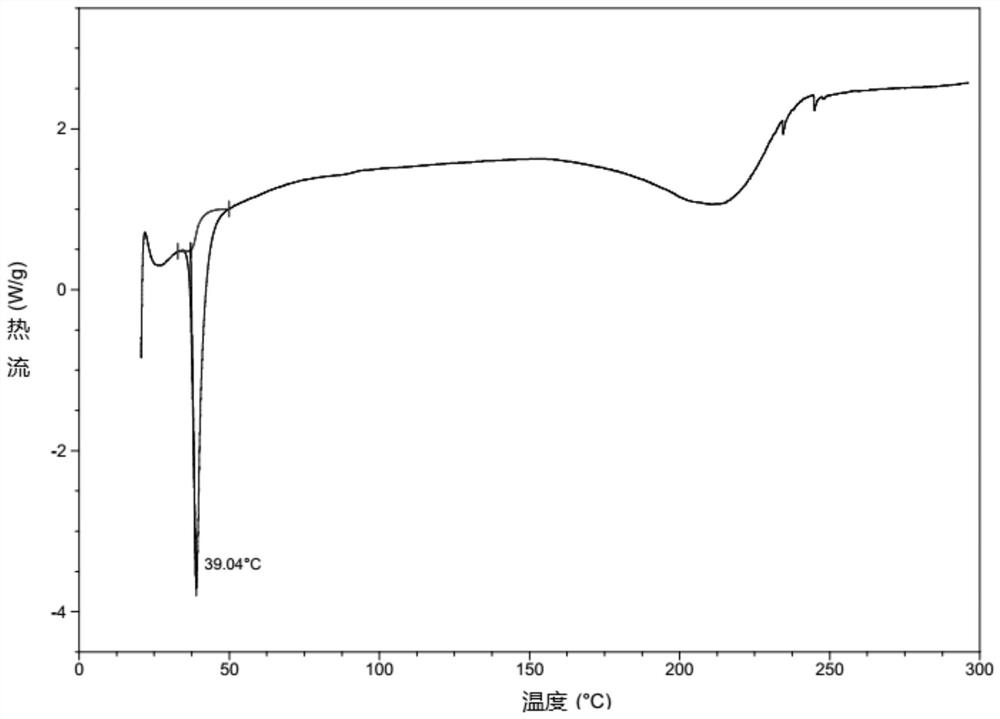
[0147] Add 1.0 g of ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methyl palmitate into 10 mL of methanol and 10 mL of acetonitrile , stirred at room temperature to obtain a clear solution, cooled to 0°C, a solid was precipitated, filtered with suction and vacuum-dried in a drying oven at room temperature to constant weight to obtain 0.85 g of crystals. The obtained crystals were confirmed to be Form A by XRD and DSC detection.
Embodiment 2
[0148] Example 2: Preparation of ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methyl palmitate crystal form A
[0149] Add 1.0 g of ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methyl palmitate to 10 mL methanol and 10 mL trifluoro In ethanol, stirred at room temperature to obtain a clear solution, cooled to 0°C, a solid precipitated, filtered with suction and vacuum-dried at room temperature in a drying oven to obtain 0.88 g of crystals. The obtained crystals were confirmed to be Form A by XRD and DSC detection, see attached figure 1 - attached image 3 .
Embodiment 3
[0150] Example 3: Preparation of ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methyl palmitate crystal form A
[0151] Add 500 mg of ((((1r,3R,5S,7r)-3,5-dimethyladamantan-1-yl)carbamoyl)oxy)methyl palmitate into 5 mL of ethanol to obtain a clear solution, Then 10 mL of water was added dropwise, and a solid precipitated out, which was filtered by suction and vacuum-dried in a drying oven at room temperature to obtain 430 mg of crystals. The obtained crystals were confirmed to be Form A by XRD and DSC detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com