Recombinant human-like elastin and composition thereof
A technology of elastin and recombinant protein, applied in the field of recombinant protein, can solve the problems of protein folding error, small protein molecular weight and easy degradation, and inability to effectively prevent elastin degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: The technical scheme for the construction of engineering bacteria expressing recombinant human-like elastin is as follows:
[0031] 1. Design of the target protein sequence: Human elastin-like protein (ELP) is a recombinant protein obtained by heterologous expression with an artificial sequence designed based on the amino acid sequence of tropoelastin. Usually in the form of (VAPGVG) 3 The peptide chain composed of S amino acid sequence repeat sequence is better than tropoelastin in animals in terms of structure and function. In this embodiment, the protein sequence is designed to repeat 5 times, namely [(VAPGVG) 3 S] 5 , as shown in SEQ NO.1.
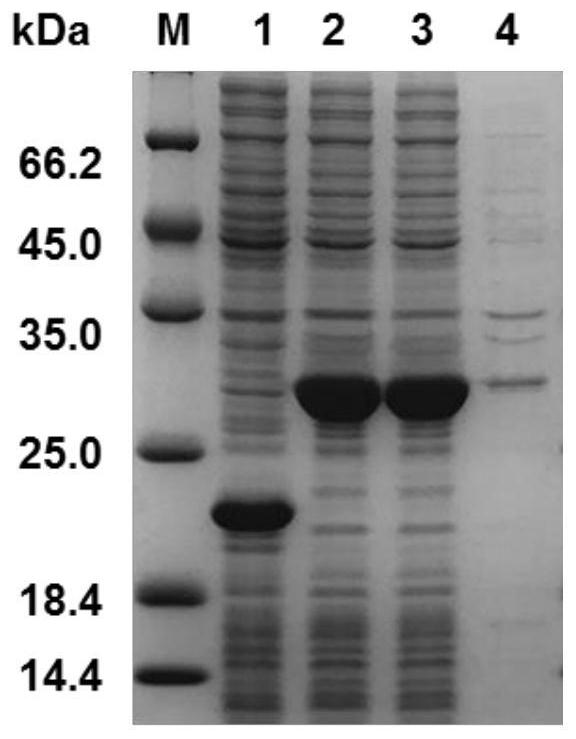
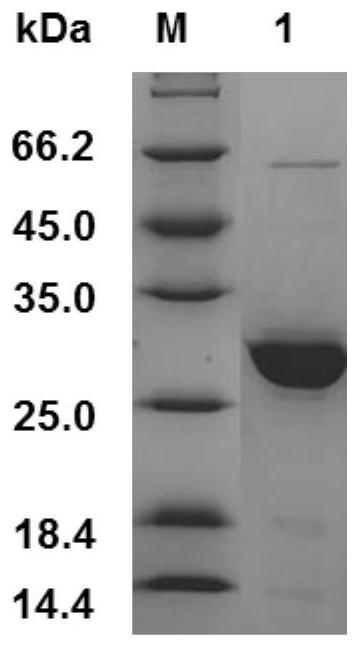
[0032] 2. Acquisition of the target gene: the recombinant human elastin gene (rhELP) was artificially synthesized by General Biosystems (Anhui) Co., Ltd., and the target sequence was optimized according to the codon preference of Escherichia coli. Insert restriction sites BamH I (GGATCC) and Hind III (AAGCTT) respe...
Embodiment 2
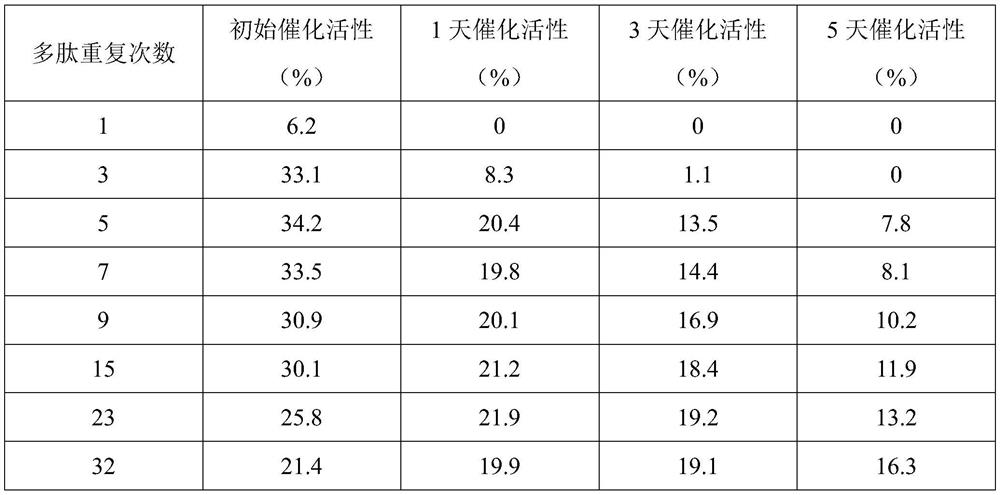
[0038] Embodiment 2: all the other are identical with embodiment 1, and difference is that (VAPGVG) 3 The number of repetitions of the S amino acid sequence was designed to be 1, 3, 5, 7, 9, 11, 15, and 32 times, and the recombinant bacteria were constructed respectively, and recombinant human-like elastin with different repetition times was purified, and the purified protein stock solution (Concentration: 0.1mg / mL) was used as the test sample, and the superoxide anion scavenging rate (ie, catalytic activity) of different recombinant proteins under different storage days was compared at a constant temperature of 37°C. The results are shown in Table 1.
[0039] Table 1 Comparison results of catalytic activity of recombinant human elastin with different repetition times
[0040]
[0041] The catalytic activity of a recombinant protein depends on the number of catalytic active sites on its surface. In theory, the more repetitions of the polypeptide active fragment on the prima...
Embodiment 3
[0044] Example 3: Dilute the recombinant human-like elastin stock solution of known concentration (concentration should be greater than or equal to 0.1 mg / ml) prepared in Example 1 to 0.1 mg / ml elastin with Tris-Nacl buffer containing 2% glycerol stock solution.
[0045] 1. Use 0.1mg / ml elastin stock solution to prepare protective solutions containing specific contents of arginine and bovine serum albumin BSA respectively. The contents of arginine and bovine serum albumin are:
[0046] 1) Arginine: 50mM / L, 100mM / L, 150mM / L, 200mM / L;
[0047] 2) Bovine serum albumin BSA (mass fraction): 0.2%, 0.5%, 1.0%, 1.5%, 2.0%.
[0048] 2. The prepared reagent was subjected to an accelerated destruction test at 37°C for a period of 7 days.
[0049] The detection operation steps are as follows: put all the samples into a 37°C incubator for accelerated destruction, take out the samples on the 0th, 1st, 3rd, 5th, and 7th day, and use the superoxide anion scavenging ability detection kit (So...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com