A kind of preparation method of aryl monosulfide compound
A technology for aryl monosulfides and compounds, which is applied in the field of preparation of synthetic aryl monosulfide compounds, can solve the problems of high requirements for reaction conditions, complex post-processing, and large environmental pollution, and achieve reduced production costs and high yields , the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
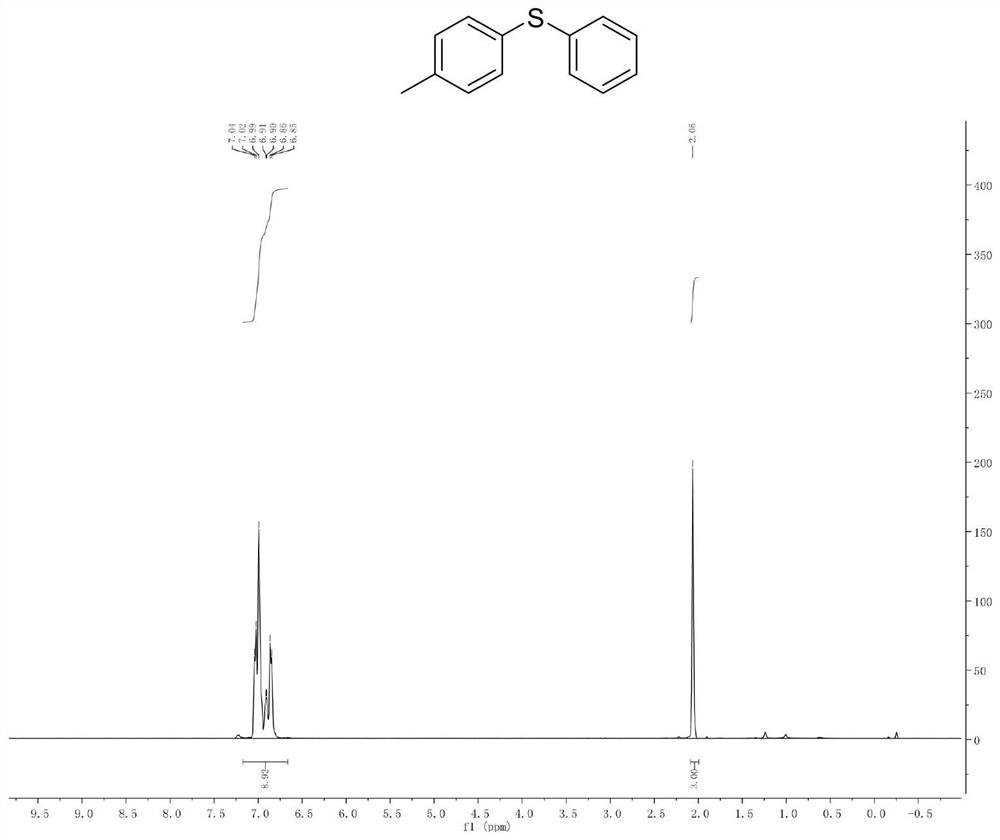
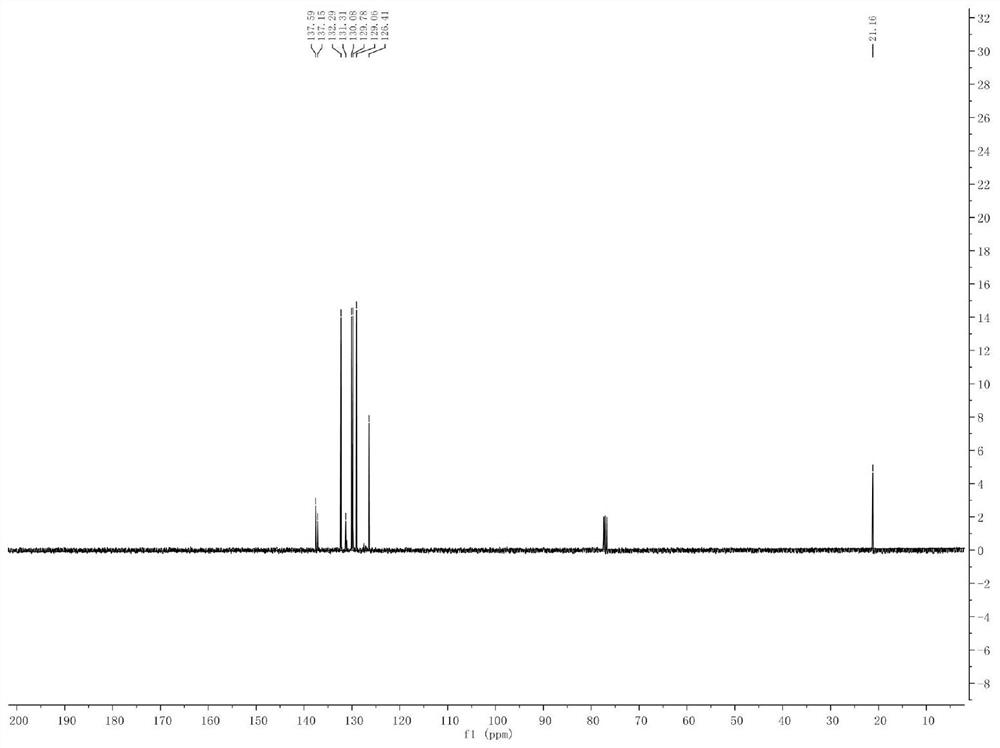
[0041] Example 1. Synthesis of Phenyl-p-Toluene Sulfide
[0042]
[0043] Add p-methyl bromide benzene (10mmol) and isopropylmagnesium chloride Grignard reagent (11mmol) to a pre-dried reaction flask filled with nitrogen (or argon), and react the mixture at -20°C for 30 minutes. The halo-magnesium exchange reaction was completely complete (reaction monitored by gas chromatography). Subsequently, the reaction solution was cooled to -78°C, and a solution of diphenyl disulfide (10 mmol) in tetrahydrofuran was slowly added dropwise to the newly prepared Grignard reagent, stirred and reacted at -78°C in an organic solvent for 1 hour, and then The reaction solution was slowly raised to room temperature. Quench the reaction with saturated ammonium chloride solution, extract the organic phase with ethyl acetate or ether, dry the organic phase with anhydrous magnesium sulfate, and concentrate the organic phase to obtain 1.6 g of a colorless oily liquid with a yield of 79% and a pu...
Embodiment 2
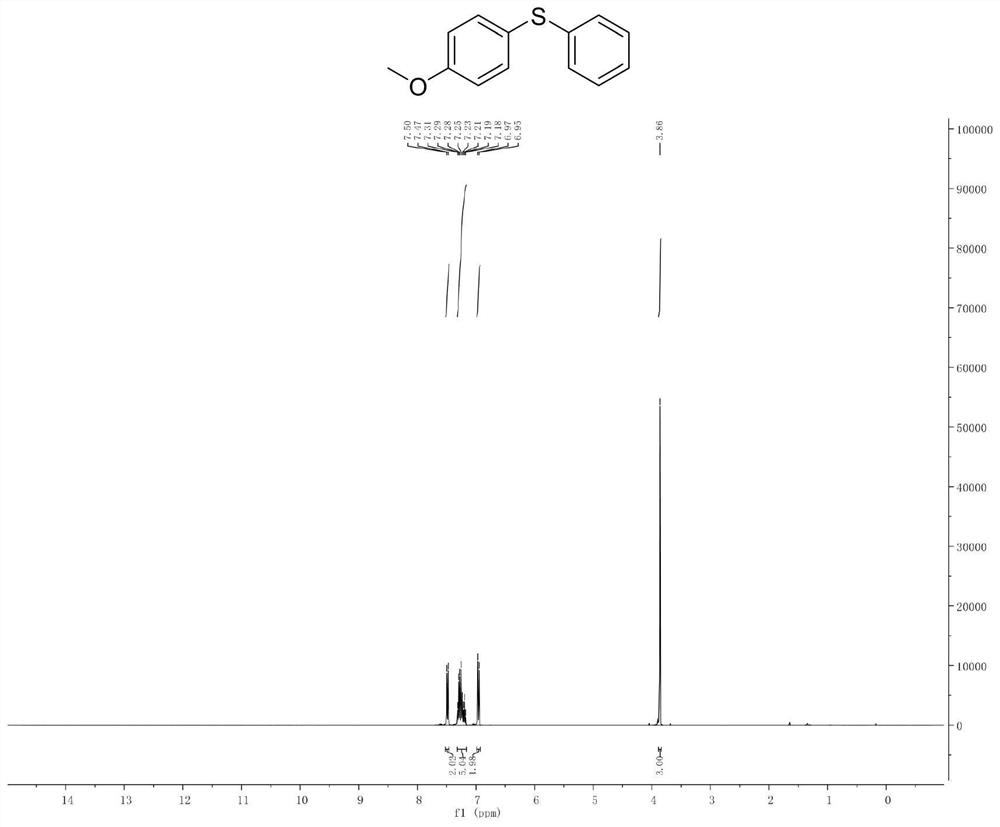
[0047] Example 2. Synthesis of phenyl p-methoxyphenyl sulfide
[0048]
[0049]Add p-methoxybromobenzene (10mmol) and isopropylmagnesium chloride Grignard reagent (11mmol) to a pre-dried reaction flask filled with nitrogen (or argon), and react the mixture at -20°C for 30 minutes. It completes the halo-magnesium exchange reaction completely (reaction monitored by gas chromatography). Subsequently, the reaction solution was cooled to -78°C, and a solution of diphenyl disulfide (10 mmol) in tetrahydrofuran was slowly added dropwise to the newly prepared Grignard reagent, stirred and reacted at -78°C in an organic solvent for 1 hour, and then The reaction solution was slowly raised to room temperature. Quench the reaction with saturated ammonium chloride solution, extract the organic phase with ethyl acetate or ether, dry the organic phase with anhydrous magnesium sulfate, and concentrate the organic phase to obtain 2.1 g of a colorless oily liquid with a yield of 95% and a ...
Embodiment 3
[0053] Example 3. Synthesis of 2,3-dihydro-5-benzofuran-p-methylphenylene sulfide
[0054]
[0055] Add 5-bromo-2,3-dihydrobenzofuran (10mmol) and isopropylmagnesium chloride Grignard reagent (11mmol) to a pre-dried reaction flask filled with nitrogen (or argon), and the mixture is kept at -20 °C for 30 minutes, until the halogen-magnesium exchange reaction is completely completed. Then the reaction solution was cooled to -78°C, and the tetrahydrofuran solution of p-methyldiphenyl disulfide compound (10 mmol) was slowly added dropwise to the newly prepared Grignard reagent, and the reaction was stirred at -78°C in an organic solvent After 1 hour, the reaction solution was slowly warmed to room temperature. Quench the reaction with saturated ammonium chloride solution, extract the organic phase with ethyl acetate or ether, dry the organic phase with anhydrous magnesium sulfate, and concentrate the organic phase to obtain a white solid 2,3-dihydro-5-benzofuran p-methyl Diph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com