Reagent for Respiratory Virus Treatment and Its Application
A respiratory and virus technology, applied in the field of respiratory virus treatment reagents, can solve the problems affecting the stability of viral nucleic acid, the impact of results, and the impact of screening efficiency, so as to save transshipment, reduce the level of protection, and improve screening efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 The composition of ready-to-use virus sampling preservation solution
[0077] Respiratory virus treatment reagent among the present invention (hereinafter referred to as ready-to-use virus sampling preservation solution, RTU) utilizes gentle nonionic detergent TWEEN20 and Nonidet P40 (NP40) to carry out cell, virus cracking, and by using dithiothreitol DTT enhanced inhibition of related nucleases such as RNase activity.
[0078] 1×RTU Composition:
[0079]
[0080] Within the above concentration range, RTU can stably perform its function.
Embodiment 2
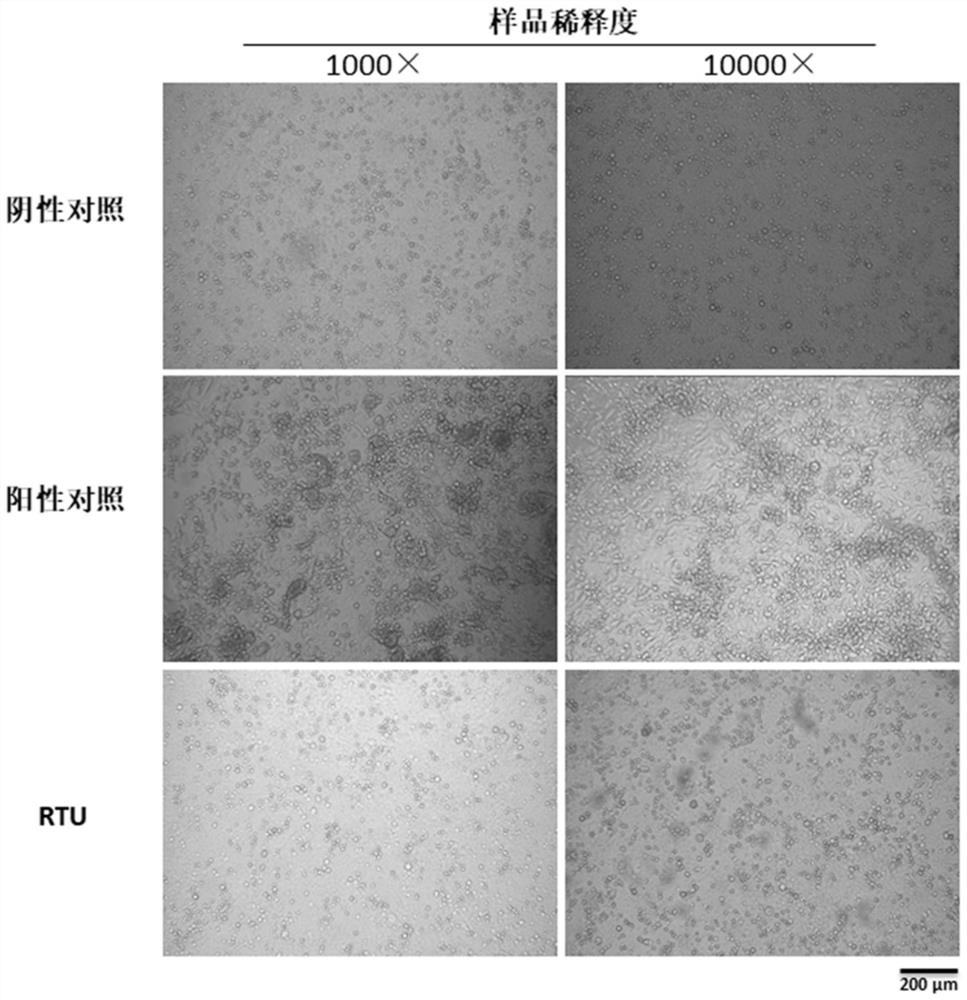
[0081] Example 2 Ready-to-use nucleic acid detection of virus isolation cell samples and throat swab samples preserved in RTU and compatibility of conventional nucleic acid extraction kits
[0082] In the experiment, 1×RTU was used to lyse the cultured and isolated cells of ADV (DNA virus) and RSV (RNA virus) kept in the laboratory, and compared with conventional commercial nucleic acid extraction kits, the virus fluorescence quantitative PCR reagent was used for detection and evaluation RTU ready-to-use, and evaluate the compatibility of RTU-preserved samples with conventional commercial nucleic acid extraction kits.
[0083] 1. Virus isolation cells preserved in RTU can be directly used for virus nucleic acid detection and compatible with conventional nucleic acid extraction kits
[0084] (1) ADV and RSV culture
[0085] ADV culture A549 cells are cultured in DMEM+5% FBS based on 37°C 5% CO2 incubator; after the cells are full, they are digested and inoculated into 96-well ...
Embodiment 3
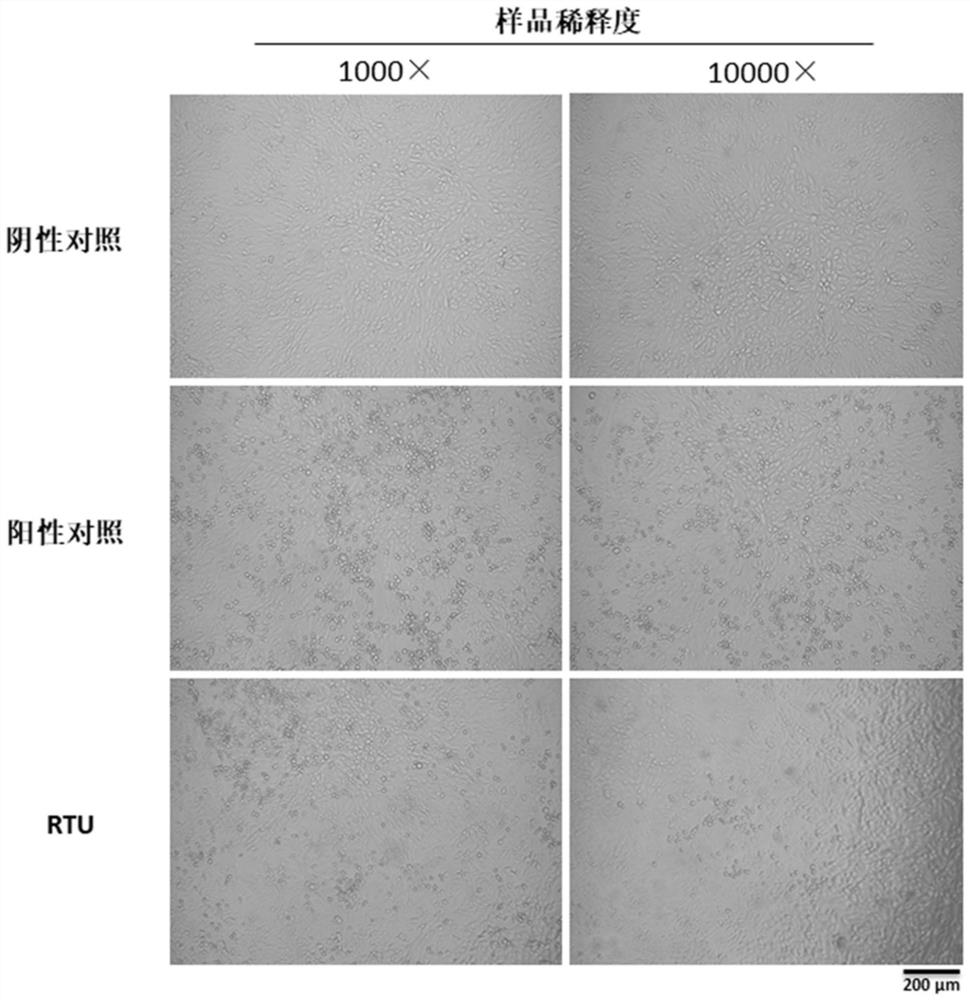
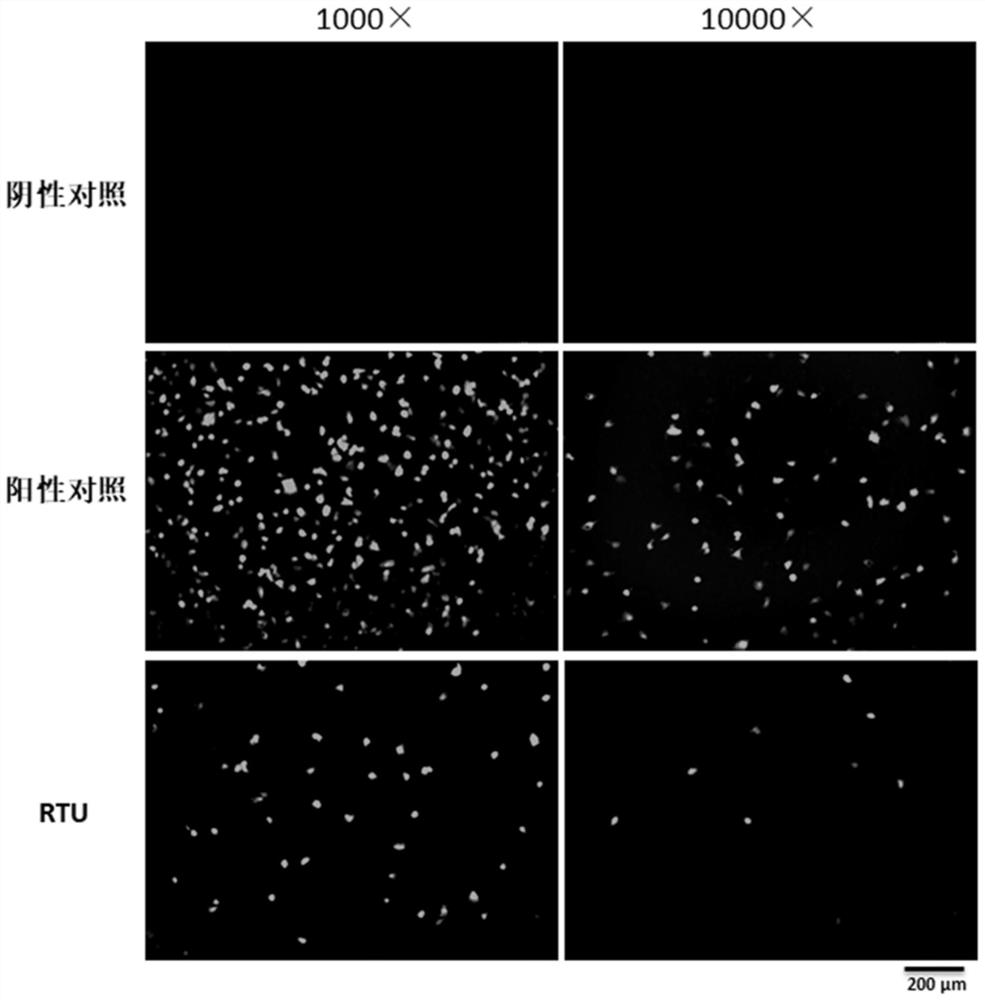
[0114] Example 3 RTU-preserved samples are compatible with antigen detection experiments
[0115] In order to verify whether the RTU-preserved samples are compatible with antigen-antibody analysis, the experiment carried out antigen detection on the RTU-preserved ADV samples.
[0116] (1) Culture of ADV
[0117] A549 cells were incubated in DMEM+5%FBS at 37°C in 5%CO 2 Cultivate in an incubator; cells are digested and inoculated into 96-well plates after the cells are full; the medium is removed when the cell density is about 80%, and the ADV virus seeds prepared in DMEM+2% FBS are inoculated at 35°C 5% CO 2 Cultured in the incubator for 2 days.
[0118] (2) Sample collection
[0119] After 2 days of virus culture, after aspirating the medium:
[0120] Use 1×RTU 100μl / well to lyse the cells and collect them into a 1.5ml centrifuge tube, vortex at high speed for 10sec, and let stand for 5min for virus antigen detection. The experiment is set in 3 parallels;
[0121] Use 1×...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com